Yohimbine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
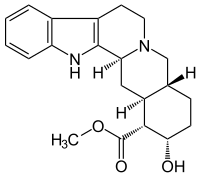
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Yohimbine | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 21 H 26 N 2 O 3 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 354.44 g · mol -1 | |||||||||||||||||||||
| Melting point |
|
|||||||||||||||||||||
| solubility |
little in water (277 mg l −1 at 25 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Yohimbine is mainly in the leaves and the bark of the yohimbe -tree ( Pausinystalia yohimbe naturally occurring) substance from the group of indole alkaloids . In addition, yohimbine can also be found in the roots of numerous snake root ( Rauvolfia species). The Rauvolfia alkaloids named after this species include not only yohimbine but also reserpine , mitragynine , serpentine and ajmaline , for example . Yohimbine is used therapeutically for erectile dysfunction .
pharmacology
Yohimbine is a highly potent antagonist of α 2 -adrenoceptors , which u. a. found in the smooth muscles of blood vessels . A blockage of these smooth muscle receptors leads to an expansion of the blood vessels. Yohimbine also quickly crosses the blood-brain barrier and increases blood pressure and heart rate through central mechanisms. It also increases motor activity and causes tremors .
Also described are an antiemetic , antidiuretic, local anesthetic and monoaminooxidase-inhibiting effect.
The aphrodisiac effect of yohimbine is said to be due on the one hand to the blockade of α 2 -adrenoceptors on blood vessels in the male sexual organs and on the other hand to the blockage of α 2 -adrenoceptors in the central nervous system . In addition, yohimbine interacts with numerous serotonin (5-HT) receptors .
Side effects
The most frequently observed side effects (1 to 10%) after taking yohimbine in therapeutic doses include insomnia , anxiety , restlessness, irritability, headache , nausea and increased urination . Occasionally (0.1 to 1%) nervousness , dizziness , vomiting , loss of appetite , stomach problems, diarrhea , sweating, shivering, palpitations, increased blood pressure and heart rate occur. The occasional occurrence of allergic reactions and skin reddening has also been reported. Significantly less common side effects include hypotension , bronchospasm and tremor . After taking a very high dose of yohimbine (200 mg), lupus erythematosus with chronic kidney failure has been described. Yohimbine can trigger panic attacks and flashbacks in patients with post-traumatic stress disorder .
Therapeutic value
Studies show that patients with psychogenic and / or organic impotence can partially benefit from medication with yohimbine. The application took place over a period of 2 to 10 weeks. A disadvantage is the strongly fluctuating bioavailability of yohimbine between 7 and 87% , which is observed between and intra-individually. With the advent of the effective PDE-5 inhibitors for the treatment of erectile dysfunction, the importance of the weak and unreliable acting yohimbine has receded.
The previously practiced treatment of high blood pressure with yohimbine has long been obsolete.
Pharmaceutical information
Yohimbine can be used orally . Yohimbine hydrochloride is used medicinally .
Veterinary medicine
In veterinary medicine, yohimbine is used to counteract the effects of xylazine in dogs and deer . In Germany, yohimbine is not approved in veterinary practice, here atipamezole is used.
Trade names
Yocon-Glenwood (D, A), Procomil (D)
Individual evidence
- ↑ a b c Entry on yohimbine in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Chemistryworld.de: YOHIMBINE AND YOHIMBINE HYDROCHLORIDE .
- ↑ a b Data sheet Yohimbine hydrochloride from Sigma-Aldrich , accessed on April 25, 2011 ( PDF ).
- ↑ Yohimbine . In: Lexicon of Biology / Chemistry online.
- ↑ Yohimbine: Potency from the equator In: Pharmazeutische Zeitung .
- ↑ van Brummelen P Vermey P, Timmermans PB, van Zwieten PA: Preliminary evidence for a postsynaptic a2-adrenoceptor in the vasculature of the human forearm . In: Br J Clin Pharmacol . 15, 1983, pp. 134P-135.
- ↑ entry to yohimbine in Vetpharm, accessed on 22 June 2012 found.
- ↑ technical information Yocon-Glanwood. Glenwood GmbH, as of February 2010.
- ↑ Steven Gabardi et al .: A Review of Dietary Supplement-Induced Renal Dysfunction . In: Clin J Am Soc Nephrol . No. 2 , 2007, p. 757-765 ( asnjournals.org ).
- ↑ Jonathan Shay, MD, Ph.D .: About Medications For Combat PTSD ( Memento of May 6, 2008 in the Internet Archive ) As of April 26, 2008.
- ^ E. Ernst E, MH Pittler: Yohimbine for erectile dysfunction: a systematic review and meta-analysis of randomized clinical trials . In: J Urol . , 1998 Feb, 159 (2), pp. 433-436, PMID 9649257 .
- ↑ Yohimbine for erectile dysfunction update ( Memento from 20091205232510) Publication by Bandolier, April 1, 2007.
- ↑ W. Blaschek, R. Hänsel, K. Keller: Hagers Handbuch der pharmaceutical Praxis , Volume 2. 5th Edition, Verlag Springer, 1998, p. 323.
- ↑ I. Knebel: Tadalafil and Vardenafil. In: Pharmazeutische Zeitung , issue 11, 2004.
- ↑ C. Leiber, U. Wetterauer: Individual Therapy for Secret Suffering In: Pharmazeutische Zeitung , Edition 37, 2005.
- ↑ CFR - Code of Federal Regulations Title 21
- ↑ W. Erhardt, J. Henke, J. Haberstroh: Anesthesia & Analgesie bei Klein- und Heimtier , Schattauer, 2004, p. 30.