Harmaline
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
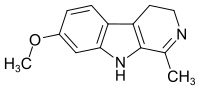
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Harmaline | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 13 H 14 N 2 O | ||||||||||||||||||
| Brief description |
beige solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass |
|
||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
232-234 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Harmalin is a psychoactive indole alkaloid from the group of Harman alkaloids . It is the reduced ( hydrogenated ) form of harmine .
history
In 1841 harmaline was from the Syrian rue isolated from Goegel. In 1919 Perkin Jr. (son of WH Perkin ) and Robinson proposed a structure, which was underpinned in 1927 by the first synthesis of Manske, Perkin and Robinson. In the same year Hasenfratz published an improved extraction method for harmaline.
Occurrence
The plants Banisteriopsis caapi (a jungle tendril) and Peganum harmala ( rue ) as well as Ayahuasca contain harmaline.
effect
Harmaline has a oneirogenic effect ( dream-generating ) and causes ataxia . It differs significantly from hallucinogens in its pharmacology . Harmalin is a reversible monoamine oxidase inhibitor and can sometimes enormously or uncontrollably increase the effect of some drugs. Harmaline does not create physical or psychological dependence .
Web links
- Harmaline and harmine . In: Erowid . (English)
- TIHKAL, # 13 (English)
Individual evidence
- ↑ a b c data sheet Harmaline at Sigma-Aldrich , accessed on December 22, 2013 ( PDF ).
- ↑ Lotsof, HS "Method of treating chemical dependency using β-carboline alkaloids, derivatives and salts thereof" United States Patent US5591738 1997 .
- ↑ Perkin, WH, Jr .; Robinson, R. "LXXIX. Harmine and harmaline. Part III." Journal of the Chemical Society , Transactions 1919 , 115 , 933-967.
- ↑ Perkin, WH, Jr .; Robinson, R. "LXXX. Harmine and harmaline. Part IV." Journal of the Chemical Society, Transactions 1919 , 115 , 967-972.
- ↑ Manske, RHF; Perkin, WH, Jr .; Robinson, R. "Harmine and Harmaline. Part IX. A Synthesis of Harmaline." Journal of the Chemical Society 1927 , 1-14.
- ↑ Hasenfratz, V. "Harmaline and harmine." Annali di Chimica Applicata 1927 , 7 , 151-226.

