Thrombin
| Thrombin | ||
|---|---|---|
|
Existing structural data: see UniProt entry |
||
| Properties of human protein | ||
| Mass / length primary structure | 70 kilodaltons / 622 amino acids | |
| Secondary to quaternary structure | Heterodimer | |
| Cofactor | Ca 2+ | |
| Precursor | Prothrombin; 579 AS | |
| Identifier | ||
| Gene names | F2 ; PT | |
| External IDs | ||
| Drug information | ||
| ATC code |
B02 BC06 B02 BD30 |
|
| Enzyme classification | ||
| EC, category | 3.4.21.5 , serine protease | |
| MEROPS | S01.217 | |
| Response type | hydrolysis | |
| Substrate | Fibrinogen | |
| Products | Fibrinopeptide A + fibrinopeptide B + fibrin precursor | |
| Occurrence | ||
| Homology family | Thrombin | |
| Parent taxon | Euteleostomi | |
| Orthologue | ||
| human | House mouse | |
| Entrez | 2147 | 14061 |
| Ensemble | ENSG00000180210 | ENSMUSG00000027249 |
| UniProt | P00734 | P19221 |
| Refseq (mRNA) | NM_000506 | NM_010168 |
| Refseq (protein) | NP_000497 | NP_034298 |
| Gene locus | Chr 11: 46.72 - 46.74 Mb | Chr 2: 91.63 - 91.64 Mb |
| PubMed search | 2147 |
14061
|
Thrombin (factor IIa) the most important is the enzyme of the coagulation in vertebrates, and one of the blood clotting factors . Thrombin belongs to the serine proteases and splits fibrinogen into fibrin and the fibrinopeptides. It also activates coagulation factors V , VIII , XIII and, together with thrombomodulin , the protein C . It has functions in the inflammatory process and in wound healing. Thrombin is formed in the liver and is found continuously as a precursor (prothrombin) in the blood plasma . Defects in the F2 gene are the cause of dysprothrombinemia and can increase the susceptibility to stroke .
Occurrence
Prothrombin is factor II in blood clotting. It is created in the liver and is continuously released into the blood. Prothrombin can be detected in the blood plasma . In the blood only small traces free thrombin, which normally only when find injury of tissue is formed from the prothrombin in place. Thrombinemia, the occurrence of free thrombin and thus the coagulation of blood in uninjured vessels, is prevented by the body's own antithrombin .
biosynthesis
In humans, the F2 gene is located on chromosome 11 and extends over 20,300 base pairs and 14 exons . After transcription , the 1,997 base long mRNA is translated into a 622 amino acid long protein and post-translational modification results in prothrombin with 579 amino acids.
Biological function
The effect of thrombin is based on its protease activity and relates on the one hand to the proteolytic activation of proteins dissolved in the blood plasma (coagulation factors and fibrin ) and on the other hand to the signaling effect on vascular cells.
Damage to a blood vessel triggers a proteolytic reaction cascade in the blood plasma, at the end of which there is the release of thrombin from prothrombin. FIIa generation takes place in the prothrombinase complex (coagulation factors II, Va and Xa, possibly associated with membrane phospholipids via calcium ions ). The released thrombin splits the fibrinogen dissolved in the blood plasma into fibrin, which aggregates to form insoluble fibrin polymers. At the same time, thrombin promotes the activation of coagulation factors V, VIII and XI and thus - via a positive feedback loop - its own release.
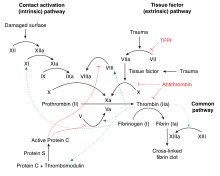
In addition to the effect of thrombin on plasma proteins, there is also its signaling effect on cells of the vascular system. The mediation of this signal effect takes place via protease-activated receptors (PAR) on the surface and the like. a. of platelets and smooth muscle cells. In thrombocytes, thrombin triggers platelet activation, in vascular smooth muscle cells proliferation and migration processes, which are also important for atherosclerotic processes.
Pharmacology, diagnosis
Thrombin can be inhibited by numerous active substances, e.g. B .:
- Antithrombin
- Heparin
- Low molecular weight heparins
- Pentasaccharide
- Orgaran
- Argatroban
- Hirudin
- Rhodniin
- Ornithodorine
- Dabigatran etexilate
The function of the thrombin can be assessed using various coagulation tests: Prothrombin level, thromboplastin time ( Quick value ), partial thromboplastin time , thrombin generation test.
Applications in the food industry
Thrombin from domestic pigs ( Sus scrofa ) is used by the food industry in the production of foods such as sausages. In May 2010 , the European Parliament rejected its application as an adhesive to artificially produce larger pieces of meat such as ham .
See also: meat glue
history
Thrombin was first described by Schmidt in 1892 in his work on blood theory:
" The coagulation of fibers has a number of reactions in the circulating blood to its more distant prerequisite and consists essentially (...) in three consecutive and interdependent acts, namely:
- in the cleavage of thrombin from prothrombin by the cytoplastic substances, which continues with increased strength.
- In the action of thrombin, which is involved in the cleavage of paraglobulin (fibrinoplastic substance) and the conversion of the fibrinogenic substance resulting from this cleavage into the liquid fiber and
- in the precipitation of the latter by the plasma salts in an insoluble modification. "
In 1946, K. Lenggenhager and in 1956 also KEA Schmidt reported that the wounds did not bleed as much during surgery if they were in the presence of thrombin. In 1956, H. Harnisch also reported on its use in dentistry . It was used in further surgery from the 1950s.
Individual evidence
- ↑ UniProt entry
- ↑ ENSEMBL entry
- ^ Stouffer GA, Runge MS: The role of secondary growth factor production in thrombin-induced proliferation of vascular smooth muscle cells . In: Semin. Thromb. Hemost. . 24, No. 2, 1998, pp. 145-50. PMID 9579635 .
- ↑ spiegel online: Industry must do without glue (accessed on May 19, 2010).
- ↑ "European Parliament stops meat paste" ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. (Süddeutsche Zeitung of May 20, 2010).
- ↑ European Parliament Press Release: MEP's veto "meat glue" authorization .
- ^ Wolf-Dieter Müller-Jahncke , Christoph Friedrich , Ulrich Meyer: Medicinal history . 2nd, revised and expanded edition. Wissenschaftliche Verlagsgesellschaft mbH, Stuttgart 2005, ISBN 978-3-8047-2113-5 , p. 116 f .
Web links
- AXEL W. BAUER and KERSTIN MALL: HEMOSTASIS, THROMBOSIS AND EMBOLIA - Historical Concepts on the Physiology and Pathology of Blood Coagulation ( Memento from June 19, 2008 in the Internet Archive )
- Schmidt: To the blood theory. Leipzig 1892 in the internet archive