Hirudin
| Hirudin-1 ( Hirudo medicinalis ) | ||
|---|---|---|
|
||
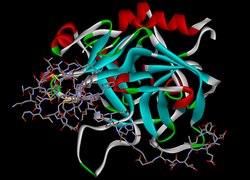
| Model of hirudin (rods) in complex with thrombin (ribbons) according to PDB 4HTC | ||
|
Existing structural data: s. UniProt |
||
| Mass / length primary structure | 65 amino acids | |
| Identifier | ||
| External IDs | ||
| Drug information | ||
| ATC code | B01 AE01 , B01 AE02 | |
| DrugBank | DB00001 | |
| Drug class | Fibrinolytic | |
| Inhibitor classification | ||
| MEROPS | I14.001 | |
| Occurrence | ||
| Parent taxon | Hirudo medicinalis | |
Hirudin is a mixture of chemically very similar polypeptides from the saliva of the medicinal leech ( Hirudo medicinalis ) with anticoagulant ( anticoagulant ) properties. They belong to the pharmacological group of the peptide thrombin inhibitors.
In 1884 the British physiologist John Berry Haycraft (1857–1922) discovered that leeches secrete a substance with a strong anticoagulant effect when sucking blood, which suppresses blood clotting at the bite site, and which Jakoby called hirudin in 1904 . The substance was first isolated in 1955 by the pharmacologist Fritz Markwardt by extraction from leech heads.
Chemical structure
The hirudin polypeptides are linearly composed of 65 to 66 amino acids , have 3 disulfide bridges and have a sulfated tyrosine residue in position 63. The isoelectric point is around pH 3.9. The complete amino acid sequence of hirudin variant-1 (HV-1) was described in 1984.
Medically used forms of hirudin are native hirudin (obtained from the head and throat ring of the leech; also by attaching live leeches directly to the patient, mainly in naturopathy ) and recombinant hirudin (r-hirudin) produced by genetic engineering . Such r-hirudins obtained from genetically modified yeasts ( Saccharomyces cerevisiae ) are lepirudin and desirudin .
| Surname | CAS | Alternate name | description |
|---|---|---|---|
| Hirudin | 8001-27-2 | - | native hirudin peptides |
| Desirudin ( INN ) | 120993-53-5 | 63-desulfohirudin | recombinant isoform HV-1 |
| Lepirudin (INN) | 138068-37-8 | 1-Leu-2-Thr-63-desulfohirudin | recombinant isoform HV-1 |
Mechanism of action and areas of application
Hirudin binds to the fibrinogen binding site of thrombin and inhibits the active center via a branch , thereby blocking its effect. r-Hirudin was used to inhibit coagulation after heparin-induced thrombocytopenia of type II (HIT II). The external use of native hirudin in the form of ointments and gels to reduce blood clotting in superficial thromboses , thrombophlebitis and bruises ( hematomas ) is no longer common.
Hirudin crosses the placenta barrier and passes into breast milk .
The therapy with hirudin can be controlled by determining the PTT . Since there is no specific antidote available in case of is overdosing a hemofiltration or hemodialysis is required.
Finished medicinal products
- Lepirudin: was available in the EU as a finished medicinal product (powder for solution for injection) under the name Refludan . The active ingredient manufacturer gave up production, so that the sale of Refludan was discontinued in April 2012 after the risk-benefit assessment was questioned by an EPAR in 2009 .
- Desirudin : Revasc (D, A)
- Bivalirudin : Angiox®
literature
- G. Nowak, K. Schrör: Hirudin - the long and stony way from an anticoagulant peptide in the saliva of medicinal leech to a recombinant drug and beyond. A historical piece. In: Thromb. Haemost. Volume 98, 2007, pp. 116-119. PMID 17598001 doi: 10.1160 / TH07-05-0364 PDF
Web links
- Public Assessment Report (EPAR) of the European Medicines Agency (EMA) for: Lepirudin
- Public Assessment Report (EPAR) of the European Medicines Agency (EMA) for: Desirudin
Individual evidence
- ↑ to the peptidic thrombin inhibitors , so that: bivalirudin (Angiox® except trade), Desirudin , Hirudin (from the blood flukes ), lepirudin (Refludan® except trade). Compared to the non-peptide thrombin inhibitors, these include: Argatroban (Argatra®), Dabigatran (Pradaxa®, antidote : Idarucizumab (Praxbind®)), Ximelagatran (Exanta®, except for commercial, hepatotoxic), prodrug of Melagatran .
- ↑ JB Haycraft: About the action of a secretion of the official leech on the coagulability of the blood. In: Arch. Exp. Pathol. Pharmacol. Volume 18 1884, pp. 209-217.
- ^ C. Jacoby: About Hirudin. In: German Medical Weekly. 30, 1904, pp. 1786-1794.
- ↑ W. Forth, D. Henschler: General and special pharmacology and toxicology. 8th edition. 2001, p. 568.
- ^ Fritz Markwardt : Studies on Hirudin. In: Natural Sciences. Volume 42, 1955, pp. 537-538. doi: 10.1007 / BF00630151
- ↑ E. Teuscher: Biogenic Medicines. 5th edition. Wissenschaftliche Verlagsgesellschaft, 1997, ISBN 3-8047-1482-X , p. 399.
- ↑ J. Dodt et al: The complete amino acid sequence of hirudin, a thrombin specific inhibitor application of color carboxymethylation. In: FEBS Lett . Volume 165, 1984, pp. 180-184. doi: 10.1016 / 0014-5793 (84) 80165-9
- ↑ UniProt P01050
- ↑ EPAR