3,4,5-trimethoxyamphetamine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
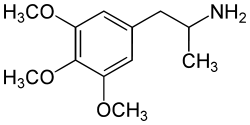
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Trimethoxyamphetamine | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 12 H 19 NO 3 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 225.29 g mol −1 | |||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
3,4,5-Trimethoxyamphetamine ( TMA ) is a psychedelic hallucinogen in the phenethylamine family . It was first synthesized in 1948 by chemist P. Hey at the University of Leeds before it was rediscovered and resynthesized by Alexander Shulgin in 1960.
TMA is seldom available, much rarer than TMA-2 or TMA-6 (which seems logical because of the much lower potency and lower-yielding sales), it has already been sold as TMA and "mescaline pep" (ie as mescaline ) at Freetekno or other parties and amphetamine mixture). In mid-2005 the Austrian criminal police seized a large amount of TMA in the Lower Austria area, which was intended for sale.
TMA is a non-marketable narcotic drug in Germany ( BtMG , Annex I ).
chemistry
Chemically, TMA is the amphetamine analogue of mescaline (3,4,5-trimethoxyphenethylamine). It is almost twice as potent as mescaline, which can be attributed to the alpha- methyl group, which prevents it from being broken down too quickly by monoamine oxidase (MAO). As a rule, the amphetamine analogues are more potent than the respective phenethylamines. A methoxy group at the 2-position (instead of the 3-position in TMA) of the phenyl ring leads to an increase in potency (probably also by inhibiting the MAO), as can be seen in the analogues TMA-2 and TMA-6.
pharmacology
TMA unfolds its effect via the noradrenergic, serotonergic and dopaminergic system; there is hardly any information available in detail. The duration of action is between 6 and 8 hours, depending on the dose.
application
TMA is usually taken orally , but it can also be taken up through the nasal mucosa. With the structurally related 2C-T-7 , this form of consumption has already resulted in deaths and thus the substance has been banned.
Web links
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
