Dimethyltryptamine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
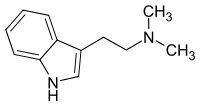
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Dimethyltryptamine | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula |
|
|||||||||||||||||||||
| Brief description |
|
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass |
|
|||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1.096 g cm −3 (base) |
|||||||||||||||||||||
| Melting point | ||||||||||||||||||||||
| pK s value |
8.68 |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
N , N -Dimethyltryptamin just DMT is a hallucinogenic tryptamine - alkaloid . It finds u. a. Use as a psychedelic or entheogen by smoking it (as a free base), sniffing it (as a yopo ) or injecting it (as a fumarate salt). An effect with peroral intake ( e.g. as ayahuasca ) is only possible with simultaneous or previous intake of monoamine oxidase inhibitors ( MAOH or MAOI for short ), as DMT isbroken downvery quickly ( first pass effect ) by the body's own enzyme monoamine oxidase (type A) becomes.
Dimethyltryptamine was first synthesized in 1931 by the Canadian chemist Richard Helmuth Fredrick Manske (1901–1977) .
Effects
When supplied exogenously, DMT has an intensive effect on the visual cortex of the brain and leads to a pronounced change in visual experience. As a rule, the consumer remains aware of the fact that he is intoxicated and, in the strict sense, is not subject to hallucinatory hallucinations, but rather extreme forms of pseudo-hallucinations . Higher dosages sometimes lead to the experience of other realities that are perceived as real. Consumption can lead to near-death experiences . Oral ingestion, combined with MAO inhibitors, usually leads to vomiting , sometimes accompanied by diarrhea .
pharmacology
DMT acts as a full agonist at the 5-HT 2A receptor and does not bind selectively with binding affinities <0.6 μM to the following serotonin receptors : 5-HT 1A , 5-HT 1B , 5-HT 1D , 5-HT 2A , 5-HT 2B , 5-HT 2C , 5-HT 6 and 5-HT 7 . DMT also binds to the Sigma-1 receptor , which is interpreted as an indication of its role as an endogenous neurotransmitter . In contrast to type-related hallucinogens such as LSD , psilocin and mescaline , DMT does not develop tolerance , the reason for this is unclear. Studies in rats and flies suggest that DMT promotes neuronal plasticity .
Usual dosages for intoxication purposes are in the two to three-digit milligram range ; a peroral uptake without MAOI leads to no noticeable effects.
Use and occurrence
DMT has been used in South America for millennia. It is the main active ingredient of Ayahuasca , a brew used for healing purposes by indigenous cultures of South America. Ayahuasca consists of a DMT source (mostly Psychotria viridis or Diplopterys cabrerana ) and ingredients that inhibit monoamine oxidase (mostly Banisteriopsis caapi , a type of liana).
Other plants that contain an increased amount of DMT are Mimosa hostilis , Anadenanthera peregrina , Codariocalyx motorius (telegraph plant), reed (Phragmites australis), Phalaris arundinacea ( reed grass) and other subspecies, many species of the genus Acacia and other plants. It occurs in the skin gland secretions of the cane toad and possibly also in mammals. Whether DMT is produced as "endogenous dimethyltryptamine" in some tissues, including the human organism, has not yet been conclusively scientifically proven, but some studies suggest this assumption.
Legal status
In the Federal Republic of Germany, DMT is a non-marketable narcotic due to its listing in Annex I BtMG . Handling without permission is generally a criminal offense.
literature
- Rick Strassman: DMT, The Molecule of Consciousness , AT Verlag, Baden 2004, ISBN 3-85502-967-9
- Jonathan Ott: Ayahuasca analogues: Pan-phean entheogens. 1996, ISBN 3-930442-08-6
- TM Carbonaro, MB Gatch: Neuropharmacology of N, N-dimethyltryptamine. In: Brain research bulletin. April 2016, doi : 10.1016 / j.brainresbull.2016.04.016 , PMID 27126737 (Review).
- Klaus Helm: Synthesis and functional in-vitro pharmacology of new ligands of the 5-HT 2A receptor from the tryptamine class. Dissertation, University of Regensburg 2014
- SA Barker: N, N-Dimethyltryptamine (DMT), an Endogenous Hallucinogen: Past, Present, and Future Research to Determine Its Role and Function. In: Frontiers in neuroscience. Volume 12, 2018, p. 536, doi : 10.3389 / fnins.2018.00536 , PMID 30127713 , PMC 6088236 (free full text) (review).
Web links
- DMT . In: Erowid . (English)
- isomerdesign.com: DMT (English)
- DMT: The Spirit Molecule in the Internet Movie Database (English)
- Series: Hallucinogens: DMT - The strongest psychedelic in the world. In: Spektrum.de. January 26, 2019, accessed February 5, 2019 .
Individual evidence
- ↑ Qu, Shi-Jin; Tan, Chang-Heng; Zhu, Da-Yuan; Wang, Gui-Feng; Zuo, Jian-Ping; Duan, Wen-Hu; Yao, Shan-Yan: Tryptamine derivatives as novel non-nucleosidic inhibitors against hepatitis B virus . In: Bioorganic & Medicinal Chemistry 19 (2011) 3120-3127, doi : 10.1016 / j.bmc.2011.04.004 .
- ^ A b Royal Pharmaceutical Society (ed.): Clarke's Analysis of Drugs and Poisons FOURTH EDITION . Pharmaceutical Press, London / Chicago 2011, ISBN 978-0-85369-711-4 .
- ^ R. Bergin, D. Carlström, G. Falkenberg, H. Ringertz: Preliminary X-ray crystallographic study of some psychoactive indole bases in Acta Cryst. B 24 (1968) 882, doi : 10.1107 / S0567740868003353 .
- ↑ Entry on N, N-dimethyltryptamine. In: Römpp Online . Georg Thieme Verlag, accessed on May 29, 2014.
- ↑ a b Entry on dimethyltryptamine in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ DMT data sheet (PDF; 38 kB) from Tocris Bioscience, accessed March 8, 2013.
- ↑ a b Rick Strassman: DMT, The Molecule of Consciousness , AT Verlag, Baden 2004, ISBN 3-85502-967-9 .
- ↑ Dimethyltryptamine (DMT). In: The Drug Classroom. Retrieved December 6, 2018 (American English).
- ^ DMT Models the Near-Death Experience. Front. Psychol., August 15, 2018, accessed on September 1, 2018. DOI: 10.3389 / fpsyg.2018.01424
- ↑ Smith RL, Canton H, Barrett RJ, Sanders-Bush E: Agonist properties of N, N-dimethyltryptamine at serotonin 5-HT2A and 5-HT2C receptors . In: Pharmacol. Biochem. Behav. . 61, No. 3, November 1998, pp. 323-30. doi : 10.1016 / S0091-3057 (98) 00110-5 . PMID 9768567 .
- ↑ Keizer MJ, Setola V., Irwin JJ, Laggner C., Abbas AI, Horseshoe SJ, Jensen NH et al .: Predicting new molecular targets for known drugs . In: Nature . 462, No. 7270, November 2009, pp. 175-81. doi : 10.1038 / nature08506 . PMID 19881490 . PMC 2784146 (free full text).
- ↑ Deliganis AV, Pierce PA, Peroutka SJ: Differential interactions of dimethyltryptamine (DMT) with 5-HT 1A and 5-HT 2 receptors . In: Biochemical Pharmacology . 41, No. 11, June 1991, pp. 1739-44. doi : 10.1016 / 0006-2952 (91) 90178-8 . PMID 1828347 .
- ↑ Pierce PA, Peroutka SJ: Hallucinogenic drug interactions with neurotransmitter receptor binding sites in human cortex . In: Psychopharmacology . 97, No. 1, 1989, pp. 118-22. doi : 10.1007 / BF00443425 . PMID 2540505 .
- ^ Smith RL, Canton H., Barrett RJ, Sanders-Bush E .: Agonist properties of N , N -dimethyltryptamine at serotonin 5-HT 2A and 5-HT 2C receptors . In: Pharmacology, Biochemistry and Behavior . 61, No. 3, November 1998, pp. 323-30. doi : 10.1016 / S0091-3057 (98) 00110-5 . PMID 9768567 .
- ^ TS Ray: Psychedelics and the human receptorome. In: PloS one. Volume 5, number 2, 2010, p. E9019, doi : 10.1371 / journal.pone.0009019 . PMID 20126400 , PMC 2814854 (free full text).
- ↑ Fontanilla D., Johannessen M., Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE: The Hallucinogen N, N-Dimethyltryptamine (DMT) Is an Endogenous Sigma-1 Receptor Regulator . In: Science . 323, No. 5916, February 2009, pp. 934-7. doi : 10.1126 / science.1166127 . PMID 19213917 . PMC 2947205 (free full text).
- ↑ Su TP, Hayashi T., Vaupel DB: When the Endogenous Hallucinogenic Trace Amine N, N-Dimethyltryptamine Meets the Sigma-1 Receptor . In: Science Signaling . 2, No. 61, 2009, p. Pe12. doi : 10.1126 / scisignal.261pe12 . PMID 19278957 . PMC 3155724 (free full text).
- ^ DE Nichols: Hallucinogens. In: Pharmacology & Therapeutics . Volume 101, Number 2, February 2004, pp. 131-181, doi : 10.1016 / j.pharmthera.2003.11.002 . PMID 14761703 .
- ↑ Psychedelic drugs promote neural plasticity in rats and flies. Accessed April 28, 2019 .
- ↑ Alexander Shulgin , Ann Shulgin: TiHKAL: The Continuation . Transform Press, Berkeley 1997, ISBN 978-0-9630096-9-2 , pp. 412-421 ( erowid.org [accessed September 1, 2019]).
- ↑ ML Pochettino, AR Cortella and M. Ruiz: Hallucinogenic Snuff from Northwestern Argentina: Microscopical Identification of Anadenanthera colubrina var. Cebil (Fabaceae) in Powdered Archaeological Material. Economic Botany, 1999, accessed May 20, 2019 .
- ↑ José M. Capriles, Christine Moore, Juan Albarracin-Jordan, Melanie J. Miller: Chemical evidence for the use of multiple psychotropic plants in a 1,000-year-old ritual bundle from South America . In: Proceedings of the National Academy of Sciences . May 1, 2019, ISSN 0027-8424 , p. 201902174 , doi : 10.1073 / pnas.1902174116 , PMID 31061128 ( pnas.org [accessed May 21, 2019]).
- ↑ Yasmin Anwar, Media Relations | May 6, 2019May 16, 2019: Ayahuasca fixings found in 1,000-year-old Andean sacred bundle. May 6, 2019, Retrieved May 21, 2019 (American English).
- ↑ Dennis J. McKenna, GHN Towers, et al .: Monoamine oxidase inhibitors in South American hallucinogenic plants: Tryptamine and β-carboline constituents of Ayahuasca. In: Journal of Ethnopharmacology . 10, 1984, pp. 195-223, doi : 10.1016 / 0378-8741 (84) 90003-5 .
- ↑ Barker SA, Monti JA, Christian ST: N, N-dimethyltryptamine: an endogenous hallucinogen . In: International Review of Neurobiology . 22, 1981, pp. 83-110. doi : 10.1016 / S0074-7742 (08) 60291-3 . PMID 6792104 .
- ^ Wallach JV: Endogenous hallucinogens as ligands of the trace amine receptors: a possible role in sensory perception . In: Medical Hypotheses . 72, No. 1, January 2009, pp. 91-4. doi : 10.1016 / j.mehy.2008.07.052 . PMID 18805646 .
- ↑ MA Thompson: Rabbit Lung Indolethylamine N-Methyltransferase. cDNA AND GENE CLONING AND CHARACTERIZATION. In: Journal of Biological Chemistry . 273, pp. 34502-34510, doi : 10.1074 / jbc.273.51.34502 .
- ↑ Steven A. Barker, Ethan H. McIlhenny, Rick Strassman: A critical review of reports of endogenous psychedelic N, N-dimethyltryptamines in humans: 1955-2010. In: Drug Testing and Analysis . 4, 2012, pp. 617-635, doi : 10.1002 / dta.422 .
- ↑ Jimo Borjigin, Michael M. Wang, Rick J. Strassman, Steven A. Barker, Ben Sheler: Biosynthesis and Extracellular Concentrations of N, N-dimethyltryptamine (DMT) in Mammalian Brain . In: Scientific Reports . tape 9 , no. 1 , June 27, 2019, p. 9333 , doi : 10.1038 / s41598-019-45812-w .

