Aga toad
| Aga toad | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|

Cane toad ( Rhinella marina ) |
||||||||||||
| Systematics | ||||||||||||
|
||||||||||||
| Scientific name | ||||||||||||
| Rhinella marina | ||||||||||||
| ( Linnaeus , 1758) |
The cane toad ( Rhinella marina , syn .: Bufo marinus ), also simply called aga or giant toad , is a species of amphibian from the genus Rhinella, which formerly belonged to the real toads ( Bufo ), within the toad family (Bufonidae). Large specimens of this species reach body lengths of over 22 centimeters. The cane toad is therefore one of the largest frogs in the world, along with the goliath frog ( Conraua goliath ), the Colombian giant toad ( Bufo blombergi ), the African bullfrog ( Pyxicephalus adspersus ) and the American bullfrog ( Rana catesbeiana ) .
Today the toad, originally native to the American continent, is one of the amphibians with the largest range: the cane toad was introduced to Jamaica and Barbados as early as the 19th century because it was hoped that it would keep the insect pests in the sugar cane plantations down could. This unsuccessful practice continued well into the 20th century, especially in the Pacific region. In some cases, this had considerable effects on the biological diversity of the countries and regions into which cane toads were introduced.
The toad is one of the most famous neozoa in the world . The negative ecological consequences that resulted from their artificial settlement serve today as a prime example of the enormous risks of uncontrolled and ill-considered biological pest control .
Rhinella marina is probably two different cryptospecies , which are difficult to distinguish morphologically . Several molecular genetic studies indicate that the populations of the cane toad found in the original range east and west of the Andes are two different species.
designation
Inspired by an illustration by Albertus Seba , the European toad was given the scientific species name "marinus" by Carl von Linné in 1758 . In English usage, the cane toad is commonly referred to as a "cane toad". This name reflects the fact that the species was already valued as a supposedly effective pest control in sugar cane plantations (sugar cane = "cane") in the early 19th century.
features
Appearance of the cane toad
The animal has a toad-typical, compact shape with a short-snouted, broad head, very conspicuously large, often triangular ear glands ( parotid ), visible eardrum , horizontal pupils , golden iris and dry, warty skin. There is a noticeable bulge above the eyes that converges over the muzzle. The hind limbs are relatively short so that the animals can only make small jumps or walk on all fours.
The upper side is colored gray-brown with dark spots; the underside is dirty-white and sometimes spotted. In contrast to the females, the males often have brown and yellow spots on the sides of the body and on the throat. Males also have dark oestrus calluses on the three inner fingers . In the mating phases, the warts of their skin are often keratinized, so that the females are described as "less warty or softer" compared to the males. The young animals initially have a lighter skin surface, which darkens with increasing age.
In the wild, females can reach a maximum size of 22.5 centimeters and weigh over a kilogram; Males stay smaller. The Guinness Book of Records also names specimens that have become much larger. "Prinsen" (dt. Prince ), an individual who was kept as a pet in Sweden, had a body length of 38 centimeters and a weight of 2.65 kilograms. A specimen preserved in a museum in Queensland was 15 inches long and weighed 1.36 kilograms.
The tadpoles are uniformly black in color. They either stay at the bottom of their spawning waters or gather close together on aquatic plants. In the final stage of their development, the larvae reach body lengths of up to 27 millimeters. After the metamorphosis , young cane toads are only between 5 and 10 millimeters long. At this point you still have smooth, dark brown skin. In some individuals, the skin surface shimmers reddish to copper-colored, similar to the young of the common toad ( Bufo bufo ).
Confusion with other species
A number of species belonging to the toad family that are very similar to the cane toad are found in North and Central America . Particularly in the juvenile stage, there are similarities with the southern toad ( Bufo terrestris ) Bonnaterre 1789, with Fowler's toad ( Bufo woodhousei fowleri ) Girard 1854 and with Schneider's toad ( Bufo schneideri ) Werner 1894.
- Bufo terrestris , however, has two pear-shaped protuberances in front of the parotid and, at 12 centimeters, does not reach the size of B. marinus in the adult stage .
- Bufo woodhousei fowleri has a typical cream-white stripe that runs down the throat.
- In contrast to the cane toad, Bufo schneideri also has poison glands secreting its hind legs.
Distribution areas and habitats
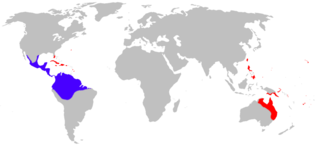
The natural range of the cane toad stretches from the Amazon region and the southeast of Peru via Central America to South Texas . In this area it primarily inhabits subtropical forests near the water as well as the tropical rainforest. However, cane toads are sufficiently adaptable to colonize a variety of other habitats, such as open grasslands, agricultural areas, wetlands of all kinds, gardens, parks and green areas of single family homes. They survive dry spells because they can lose up to 50 percent of the water content of their body without harm. Cane toads only need spawning waters to be available at least temporarily. They usually spawn in stagnant or very slowly flowing fresh water. Low salinity levels are tolerated, the maximum salinity of the spawning waters is 15 percent of the salinity concentration in seawater.
In addition to its natural range, the cane toad has been introduced by humans to numerous other warmer regions of the world. These include Australia , Papua New Guinea , the Fiji Islands, the Philippines , Taiwan , Japan , Hawaii , Florida , Puerto Rico as well as a number of smaller Caribbean islands and Mauritius .
Reproduction
As an inhabitant of tropical and subtropical habitats, the cane toad is not tied to certain seasons when it comes to reproduction. Their reproduction strategy is opportunistic, which means that the toads will always reproduce when there is enough spawning water available. A single female is able to produce between 4,000 and 36,000 eggs twice a year. The males, who have found a suitable spawning area, lure the females with a long, loud and gurgling trill. In the literature, the sound of this call is sometimes compared to the bright hum of a small, running engine.
When a female approaches, the male clasps the middle of the female's body with his front legs. This reproductive attitude , which is typical for frogs, is called the amplexus . The female then lays the spawn in the water in the form of gelatinous cords near aquatic plants. The male then inseminates the spawn externally. The clutches are mostly deposited in the shallow water zones of permanent or temporary standing water.
The single egg is black. Including the gelatinous mass that surrounds it, it measures between 4 and 5 millimeters. The time it takes for the tadpoles to hatch depends on the water temperature. In spawning waters with a temperature of 34 degrees Celsius this only takes 25 hours; if the water measures only 14 degrees Celsius, the tadpoles only hatch after 155 hours. The metamorphosis from tadpole to young cane toad also depends on the ambient temperature and takes between 12 and 60 days.
nutrition
During the day, cane toads hide under stones, leaves or tree stumps or dig into loose soil. They only catch prey at night. The food spectrum of the cane toad is very broad. To a large extent it consists of live animals which the toad can overwhelm and devour whole; mostly insects, spiders, worms and snails. The cane toad is known, for example, for crouching in front of beehives and catching the bees laden with nectar and pollen at the entrance hole. Beekeepers have found up to 300 bees in a cane toad. The cane toad also eats other amphibians and small mammals such as young mice. Cannibalism is common.
Most frogs identify their prey by moving. The cane toad can also smell food. For this reason, the range of food of the cane toad is not limited to live animals. It eats carrion and finds food in household waste . She regularly finds himself at standing outdoor food bowls for pets one. The broad spectrum of food is considered to be one of the factors that favor the successful spread of the cane toad.
Life expectancy and predators

Cane toads can live to be between 10 and 15 years old in the wild. Animals kept in captivity can live up to 20 years. In their natural range, parasites help to keep the population numbers of this species low. In addition, there are a number of predators such as the broad- snouted caiman , the snake species Leptodeira annulata and some ibises . The tadpoles are eaten by some members of the eel family , by different species of killifish , by the flag tail Kuhlia rupestris and by some species of catfish . Hunting pressure is particularly high on young cane toads. It is estimated that only 0.5 percent of young cane toads reach reproductive age.
The regions where cane toads have been released are missing the parasites that help keep population numbers low in their natural range. Among the predators in the introduction of areas, among other counts Whistling Kites ( Haliastur sphenurus ) that hydromys ( Hydromys ), the black rat and the Asian Water Monitor ( Varanus Salvator ) which is, however, itself threatened by the cane toad to other sources. There are isolated indications that owl swallows ( Podargus ) also eat cane toads. In Australia, ecologists have high hopes for the ant Iridomyrmex reburrus , which mainly attacks young toads.
Predators either have a tolerance to the poisonous secretions of the cane toads or are adapted in their behavior and eating strategies so that they only ingest small or no doses of the skin toxin.
Skin toxins
Aga toads defend themselves against potential attackers and predators with their poisonous skin secretions. The toxins are secreted both via the two large glands in the back of the ear ( parotid ) and via the skin glands on the back. In the case of significant distress, this can also be done by spraying (extrusion). The skin secretion contains several bufotoxins such as bufotenine , dimethyltryptamine (DMT) , 5-MeO-DMT , bufotaline and catecholamines ( adrenaline and noradrenaline ). This severely irritates the attacker's epidermis , eyes and oral mucous membranes. Oral contact can be fatal for mammals, birds and reptiles , depending on the amount of secretion absorbed . Dogs are reported to have died of toxins within 15 minutes of retrieving a cane toad .
In humans, too, direct contact leads to severe irritation of the mucous membranes and skin. Even deaths are now known, but only after consuming the animals or their eggs. In general, one should approach fully grown specimens in the wild with calm and caution. Caution is also advised when taking photos in the immediate vicinity of the animals.
Even the spawn and larvae of B. marinus have toxic defense substances. Cane toads show the lowest toxicity shortly after metamorphosis . At this point in time, both the posterior ear glands and the internal secretory production of skin toxins are not yet fully developed.
The cane toad as a neozoon
Settlement as part of biological pest control
Cane toads were imported into many regions of the world because it was long believed that this amphibian species could be used successfully in combating agricultural pests. The first settlements took place in 1844 on the sugar cane plantations of the Caribbean islands of Martinique , Barbados and Jamaica . In Jamaica it was intended to use the toads to combat the rat plague that ruled there , which after some time turned out to be a failure. Adult cane toads often catch and eat mice and rats, but only up to a certain size. Adult, sexually mature migrant and ship rats (syn. House rat ) can no longer be overwhelmed by the toads. This measure therefore had no effect.
In 1920, cane toads were imported into Puerto Rico . There, the toads should help reduce one of the most feared pests of sugar cane . It is the larvae of the beetle Lepidoderma albohirtum that dig into the stalks of the sugar cane plants and cause considerable damage. The settlement of the cane toad, which had spread throughout Puerto Rico as early as 1932, was actually accompanied by a striking decline in sugar cane pests at the beginning. At a scientific conference on the cultivation of sugar cane, which took place in Puerto Rico in 1932, Rhinella marina was presented so convincingly as a successful pest controller that the conference participants from Hawaii took cane toad spawn back home with them to settle. Australian sugar cane growers were also so impressed by the successes in Puerto Rico that they closely followed the attempt at introduction to Hawaii . The Puerto Rican scientists missed something important. The cause of the sharp decline in the sugar cane pest was not the settlement of the cane toad, but climatic factors. Puerto Rico experienced several periods of bad weather during this period. The years from 1931 to 1936 in particular were characterized by high amounts of precipitation alternating with unusually dry months. This weather constellation, which is unusual for Puerto Rico, considerably restricted the natural reproduction rate of the sugar cane pest. In 1943 and 1948 at the latest when the sugar cane harvest was again low due to the high level of pest infestation, despite the high population density of cane toads, it was finally clear that the introduction of the cane toad had not provided the solution to this problem. At that time, Rhinella marina had already been introduced not only to Hawaii and Australia, but also to numerous other countries because of its supposed success in pest control. Only in Egypt had the settlement failed.
Effects on the fauna of Australia
Settlement and expansion in Australia
Inspired by the positive reports from Puerto Rico and the successful naturalization in Hawaii, the first toads were introduced to Australia in 1935 by employees of a Cairns- based agricultural research institute. In 1936 another 41,800 young toads were released in Cairns, Gordonvale , Innisfail and Tully in northeast Australia. The animals were released, although critical voices warned against the settlement of this highly reproductive amphibian species as early as 1935. For a few months, the Australian government even banned the release of cane toads before giving in to demands from the owners of sugar cane plantations. Reginald Mungomery, who was responsible for the introduction, reassured the critics in 1936 by referring to the fact that the settlement in Queensland took place after carefully weighing the pros and cons and after the behavior of the toad still gives no reason to fear that we are in ours Have deceived judgment about them.
The annual population increase of B. marinus in Australia is estimated to be around 25 percent. This rapid increase is accompanied by an explosive expansion of their area of distribution. Starting from Queensland , cane toads were first observed in New South Wales in 1978 and in the Northern Territory in 1984. In autumn 2010 they reached Kununurra and the Kimberleys in Western Australia . There are now estimated to be more than 200 million cane toads in Australia (as of 2016).
The rate at which the toad population is expanding its range is estimated to be around 40 kilometers per year. The toads on the western border of their range are even reported to have developed longer hind legs. It is believed that internal population pressures allow individuals with longer legs to migrate farther to move faster into new habitats, which has nutritional and reproductive benefits.
Effects of the settlement
The Australian continent was isolated from the other continents for such a long geological period that a completely independent ecosystem has developed there that is particularly sensitive to non-native, introduced species. The introduction of a Eurocean (highly adaptable) amphibian species such as B. marinus , which has few predators due to its poisonous skin gland secretions ( bufadienolide ) and is characterized by high reproduction rates ( r-strategy ) and a wide food spectrum, inevitably had a negative impact on the sensitive ecosystem Australia have. According to current estimates, the population of B. marinus now exceeds the total number of individuals of all two hundred frog species native to Australia.
The disappearance of a number of species endemic to Australia has been directly linked to the artificial settlement of the cane toad. Not only amphibians are affected by this, but also snakes and monitor lizards , for example ; in the case of the Argus monitor ( Varanus panoptes ), for example, local populations of up to 90% were recorded after the Aga toad migrated into its habitat. The poison marten is also affected by the poisonous gland secretions of B. marinus . Natural areas into which the cane toad has penetrated show, after some time, a significant reduction or even disappearance of native monitor species . There are indications and observations that these highly developed reptiles have now learned to avoid B. marinus as prey. Snakes do not seem to have this ability to learn. For example, the snake species Acanthophis antarcticus , Pseudechis guttatus and Pseudechis porphyriacus have become very rare in regions with cane toad populations.
The Kakadu National Park , which is a World Heritage Site , is one of the regions that have been settled in recent years. Here, too, a clear impact on biological diversity is expected. The greatest risk is assessed that predators are poisoned by the cane toads. Other displacement factors, such as increased competition for resources and increased hunting pressure on native animal species by the toad, are also rated as significant, but compared to this risk as less significant.
It is not only the speed with which the cane toad is opening up new areas in Australia that is astonishing, but also the population density. It is considered the most common vertebrate species in Queensland. While in South America, even in densely populated regions of spawning waters, more than 20 adult animals per 100 meters of shoreline are rarely seen, in Queensland, Australia, 1000 to 2000 adult toads have been counted per 100 meters of shoreline.
Adaptations of predators to the toad
For some native species in Australia, the cane toad is now part of the diet. Birds of prey such as the black kite ( Milvus migrans ) have learned to concentrate on the underside of the body when attacking the toad. This avoids contact with the poison-producing glands on the back and head. In the Northern Territory, Litoria dahlii (an Australian tree frog ) appears to be able to eat both tadpoles and young toads without being harmed by the toxins. This appears to be the reason why the cane toads are spreading more slowly in some areas of the Northern Territory than had been feared. Some species of snakes are reported to have developed smaller jaws so that they are no longer able to devour large cane toads, which contain correspondingly more venom.
Scientific countermeasures
The most successful measure to date to control the cane toad is to use ultraviolet light to lure it and then kill it. In the research organization " CSIRO " (Commonwealth Scientific Industrial Research Organization), scientists recently worked on a biological weapon against the toads released by humans. A "defused" amphibian virus should be introduced into the genome of the animals, which is supposed to prevent reaching sexual maturity, which normally begins at around 12 to 18 months. In the meantime, this project had to be canceled because it turned out that native amphibians in need of protection are also negatively affected. The introduction of the parasites and viral diseases that keep the population of the cane toad low in South America had to be refrained from because adequate protection of the native frog species was not guaranteed.
In June 2006, the University of Queensland announced that it was researching a gene that would lead to sex reassignment in females of the cane toad. There are already concerns here that the spread to other amphibian species cannot be reliably prevented. There are also fears that this gene may spread to the original range of the cane toad.
One challenge is clearing up cane toad carcasses. Attempted solutions include processing them in liquid fertilizer. In the meantime, ways have been developed to process the skin of the cane toads as leather for both clothing and accessories .
In February 2011, scientists working with Daniel Florance published a study on the ratio of the aga toad to artificial water sources. They found that the spread of the cane toad in arid Australia was greatly facilitated by man-made, artificial water bodies. They propose to systematically keep toads away from such otherwise ecologically insignificant water points in order to close possible dispersal corridors for the toad. Toad fences should be built around ponds, canals, etc. - the water-dependent amphibians can then easily be collected at these fences.
Attitude of the Australians to the cane toad
Most Australians are aware of the problems that neobiota such as rabbits , water buffalo , broncos and water hyacinths cause on the Australian continent. The Australians are familiar with the particular problem of the cane toad from the 1988 documentary " Cane Toads: An unnatural history ". The film is still widely used today in environmental education in schools.
The cane toad is rejected with particular vehemence. Many Australians collect cane toads in plastic bags and then kill them by freezing them. Others beat them to death with golf or cricket clubs . In April 2005, Dave Tollner, a member of the Northern Territory Parliament, called for such killings to be legalized. However, this request has been outraged by numerous animal and nature conservation groups.
The attitude of Australians towards the cane toad has been the subject of various films and books. Morris Gleitzmann is the author of a children's book called "Toad Rage". The title is a play on words and can be translated as "toad rage" as well as "toad enthusiasm". In the book, the cane toad Limpy embarks on a journey to understand why people are so negative about cane toads. The success of the first volume inspired Morris Gleitzmann to two sequels, which appeared under the title "Toad Heaven" and "Toad away" (Toad away). The short film "Cane Toad - What happened to Baz" also deals with the attitude of the Australians towards the cane toad. The film was named Best Comedy at the 2003 St. Kilda Film Festival. The film's humor, however, assumes that one is aware of the problematic relationship between Australians and cane toads. The foreign reactions to this film were therefore much more restrained.
Cane toads as drug suppliers
The poisonous skin gland secretions of the cane toads are used by some drug users for stimulation. The "milked" secretion is dried and then inhaled through a pipe. Apart from the cruel practices that are carried out when the gland secretion is obtained, consumers expose themselves to great health risks. In addition to the hallucinogens DMT and 5-MeO-DMT , the secretion also contains numerous toxins. Consumption increases the heart rate due to the catecholamines , while the bufotoxins in turn lower the heart rate. This can lead to cardiac arrhythmias , high blood pressure and epilepsy-like cramps, among other things . It is reported that drug users lick the toads to ingest bufotenin . Bufotenin triggers mild hallucinations that should last about an hour. The secretion of the cane toad contains bufotenin only in very small amounts, while other toxins are represented in large amounts. Licking cane toads can therefore lead to serious illness and even death.
See also
Literature (selection)
- Simon Easteal: The history of introductions of Bufo marinus (Amphibia: Anura); a natural experiment in evolution. In: Biological Journal of the Linnean Society. ISSN 1095-8312 , Volume 16, 1981, pp. 93-113, doi: 10.1111 / j.1095-8312.1981.tb01645.x .
- William J. Freeland: The need to control Cane Toads. In: Search. [Australian and New Zealand Association for the Advancement of Science (ed.)], Vol. 16, Nos. 7-8, 1985, pp. 211-215.
- Walter J. Lawson: The Cane Toad ( Bufo marinus ): A Bibliography (AES working paper) . School of Australian Environmental Studies, Griffith University 1987, ISBN 0-86857-247-0 .
- Michael J. Tyler: Australian Frogs. Penguin Books, 1989, ISBN 0-670-90123-7 .
- John Barker, Gordon C. Griff, Michael J. Tyler: A Field Guide to Australian Frogs . Surrey Beatty & Sons, 1995, ISBN 0-949324-61-2 .
- Christopher Lever: The Cane Toad. The history and ecology of a successful colonist . Westbury Academic & Scientific Publishing, Otley, West Yorkshire, 2001, ISBN 1-84103-006-6 .
- Tim Low: Feral future. The untold story of Australia's exotic invaders . Penguin Books Australia, Victoria 2001, ISBN 0-14-029825-8 .
Movies
- Cane toads. The conquest of Australia. Documentary, Australia, 2015, 44:06 min., Script and director: Mark Lewis, production: Off the Fence, ZDF , 3sat , German first broadcast: 23 September 2015 on 3sat, synopsis by ARD .
- Killer Cane Toad. Documentary, USA, 2006, 52 min., Director: Pete Chinn, production: National Geographic Channel , series: National Geographic Explorer , synopsis with excerpt from National Geographic.
- Cane-Toad: What Happened to Baz? Animated film , Australia, 2002, 6 min., Written by David Clayton, James Cowen, Andrew Silke, directed by David Clayton, Andrew Silke, summary by IMDb , film page, ( Memento from August 20, 2006 in the Internet Archive ).
- Cane Toads: An Unnatural History. Documentary, Australia, 1988, 47 min., Script and director: Mark Lewis, production: Film Australia, premiere: March 21, 1988 at the New York Film Festival , first broadcast: June 9, 1988, film data from IMDb .
Web links
- Darrel L. Frost: Rhinella marina (Linnaeus 1758)
- Species portrait at Amphibiaweb.org (English)
- Rhinella marina in the endangered Red List species the IUCN 2006. Posted by: Solís et al. , 2004. Retrieved May 11, 2006.
Individual evidence
- ↑ Easteal: The history of introductions of Bufo marinus. Pp. 93-113.
- ↑ a b cone : The ant as a tramp . (= General series. No. 13282). Heyne, Munich 2001, ISBN 3-453-18439-4 , p. 153.
- ↑ Aldemar A. Acevedo, Margarita Lampo, Roberto Cipriani: The cane or marine toad, Rhinella marina (Anura, Bufonidae): two genetically and morphologically distinct species. In: Zootaxa. 4103, April 6, 2016, pp. 561-573.
- ↑ E. Wyse (Ed.): Guinness Book of Records 1998 . Guinness Publishing, 1997, ISBN 0-85112-044-X , pp. 249 .
- ↑ a b c Tim Low: Feral future. The untold story of Australia's exotic invaders . Penguin Books Australia, Victoria 2001, ISBN 0-14-029825-8 , p. 51.
- ↑ CA Ely: Development of Bufo marinus larvae in dilute sea water . In: Copeia . tape 56 , no. 4 , 1944, pp. 256 .
- ^ Tim Low: Feral future. The untold story of Australia's exotic invaders. 2001, p. 49.
- ^ Tim Low: Feral future. The untold story of Australia's exotic invaders. 2001, p. 52 f.
- ^ M. Anstis : Tadpoles of South-Eastern Australia: A Guide with Keys . Reed New Holland, 2002, ISBN 1-876334-63-0 , pp. 274 .
- ^ A heartfelt cry from the Kununurra Community to the Nation. We will Stop the Cane Toads getting into WA! . In: canetoads.com.au , 2005.
- ^ Mertens Water Monitor. (PDF; 525 kB) In: Threatened Species of the Northern Territory. Northern Territory Government, November 2006, archived from the original on March 4, 2016 ; accessed on September 3, 2019 .
- ^ R. Angus: Observation of a Papuan Frogmouth at Cape York [Queensland] . In: Australian Birds . tape 28 , 1994, pp. 10-11 .
- ↑ Ecology of Bufo marinus. Global Invasive Species Database, May 26, 2010, accessed September 27, 2015 .
- ^ Tim Low: Feral future. The untold story of Australia's exotic invaders. 2001, pp. 46-51.
- ^ Tim Low: Feral future. The untold story of Australia's exotic invaders. 2001, p. 47.
- ↑ quoted from Tim Low: Feral future. The untold story of Australia's exotic invaders. 2001, p. 46.
- ↑ Jonathan Gifford: Kununurra and Cane toads. In: ABC Perth News. September 24, 2010, accessed September 27, 2015 .
- ↑ Australia wants to process cane toads into sausage. In: Spiegel online. 15th November 2016.
- ^ MJ Tyler: Australian Frogs A Natural History . Reed Books, 1994, ISBN 0-7301-0468-0 , pp. 112 .
- ↑ John Roach: Toxic Toads Evolve Longer Legs, Study Says. In: National Geographic News. February 15, 2006, accessed May 19, 2006 .
- ↑ JS Doody et al: A Preliminary Assessment of the Impacts of Invasive Cane Toads (Bufo marinus) on Three Species of Varanid Lizards in Australia. In: Mertensiella. 16, 2007, pp. 218-227. (Advances in Monitor Research. III).
- ↑ Alina Schadwinkel: Aga toad plague in Australia: Training for the taste buds. In: time online . April 14, 2010.
- ^ Tim Low: Feral future. The untold story of Australia's exotic invaders. 2001, p. 52.
- ^ RA van Dam, DJ Walden, GW Begg: A preliminary risk assessment of cane toads in Kakadu National Park (= Supervising Scientist Report . No. 164 ). 2002 ( gov.au [accessed September 27, 2015]).
- ^ Margarita Lampo, Giulio A. De Leo: The Invasion Ecology of the Toad Bufo marinus : from South America to Australia . In: Ecological Applications . tape 8 (2) , 1998, pp. 288-296 .
- ↑ D. Mitchell, A. Jones, J.-M. Hero: Predation on the Cane Toad (Bufo marinus) by the black kite (Milvus migrans) . In: Memoirs - Queensland Museum . tape 38 , 1995, p. 512-531 .
- ↑ Emma Gumbleton: NT frog 'eats' Cane Toad. ABC News Online, archived from the original on June 3, 2007 ; accessed on February 23, 2013 .
- ↑ Ben L. Phillips, Richard Shine: Adapting to an invasive species: Toxic Cane Toads induce morphological change in Australian snakes . In: PNAS . tape 101 (49) , December 2004, pp. 17150-17155 ( pnas.org ).
- ↑ Gender bending could see cane toad's end. In: ABC news online. Archived from the original on January 25, 2007 ; accessed on February 23, 2013 .
- ^ Howard Garrett: Toads as Fertilizer. In: DirtDoctor.com. April 20, 2006, accessed September 27, 2015 .
- ↑ D. Florance et al .: Excluding access to invasion hubs can contain the spread of an invasive vertebrate. In: Proceedings of the Royal Society B . 2011. doi: 10.1098 / rspb.2011.0032 , (online pre-publication).
- ↑ wbr / dpa : Australia: fences should curb the toad plague. In: Spiegel Online . February 23, 2011, accessed December 26, 2014 .
- ↑ See Cane Toads: An unnatural history in the English language Wikipedia .
- ↑ Cane toad clubbing sparks controversy. Archived from the original on January 25, 2007 ; accessed on February 23, 2013 .
- ↑ Radar: Really caning it. Archived from the original on September 12, 2006 ; accessed on February 23, 2013 .
- ^ AT Weil, W. Davis: Bufo alvarius: a potent hallucinogen of animal origin . In: Journal of Ethnopharmacology . tape 41 (1-2) , 1994, pp. 1-8 .