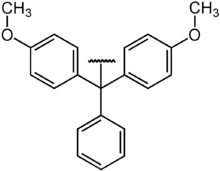
DMT protecting group
Dimethoxytrityl is a protective group for hydroxyl groups that is mainly used in nucleic acid chemistry. It is mainly used to protect the 5 'OH group of the individual nucleotides in automated DNA synthesis . The protecting group is acid labile and is normally deprotected with trifluoroacetic acid .
The protection of the single nucleotide takes place with the DMT chloride at room temperature in anhydrous pyridine , also in the presence of the 3'-OH group. DMT is so sterically demanding that the reaction takes place almost exclusively at the 5 'position (reactions on primary alcohols are generally more preferred than on secondary alcohols).

Protection of thymidine with the help of DMT-Cl.
literature
- PJ Kocieński: Protecting Groups. 1st edition, Georg Thieme Verlag, Stuttgart 1994, ISBN 3-13-135601-4 .