Palonosetron
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
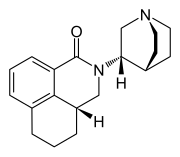
|
|||||||||||||
| General | |||||||||||||
| Non-proprietary name | Palonosetron | ||||||||||||
| other names |
2 - [( S ) -quinuclidin-3-yl] - (3a R ) -2,3,3a, 4,5,6-hexahydro-1 H -benzo [ de ] isoquinolin-1-one |
||||||||||||
| Molecular formula |
|
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| ATC code | |||||||||||||
| Drug class | |||||||||||||
| Mechanism of action |
selective blockade of central 5-HT 3 receptors |
||||||||||||
| properties | |||||||||||||
| Molar mass | |||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
87-88 ° C (palonosetron) |
||||||||||||
| solubility |
Easily soluble in water, slightly soluble in ethanol and 2-propanol (Palonosetron hydrochloride) |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Palonosetron is a in Germany in 2005 and in Austria and Switzerland under the trade name Aloxi ® (Manufacturer in Germany: Riemser drug ) for the prevention of nausea and vomiting in chemotherapy of cancer approved drug and belongs to the group of anti-emetics . As well as the already longer available ondansetron it belongs to the group of 5-HT 3 receptor antagonists, with a plasma half-life of about 40 hours, it has a much longer duration of action as ondansetron on. 250 µg palonosetron is given intravenously about 30 minutes before chemotherapy , although repeated administration within seven days is not recommended.
literature
- MS Aapro: Palonosetron as an anti-emetic and anti-nausea agent in oncology. In: Ther Clin Risk Manag. 3 (6), Dec 2007, pp. 1009-1020. PMID 18516316 , PMC 2387285 (free full text).
- NA Muchatuta, MJ Paech: Management of postoperative nausea and vomiting: focus on palonosetron. In: Ther Clin Risk Manag. 5 (1), Feb 2009, pp. 21-34. PMID 19436621 , PMC 2697527 (free full text).
Individual evidence
- ^ A b The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals. 14th edition. Merck & Co., Whitehouse Station, NJ, USA 2006, ISBN 0-911910-00-X , p. 1206.
- ↑ There is not yet a harmonized classification for this substance . A labeling of 1H-Ben [de] isoquinolin-1-one, 2- (3S) -1-azabicyclo [2.2.2] oct-3-yl-2,3,3α, 4 is shown, which is derived from a self-classification by the distributor , 5,6-hexahydro-, monohydrochloride, (3αS) - in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on July 10, 2019.
- ^ A. Russ, S. Endres (Ed.): Arzneimittelpocket Plus 2008. 4th edition. 2007, ISBN 978-3-89862-287-5 .
