Sibutramine
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
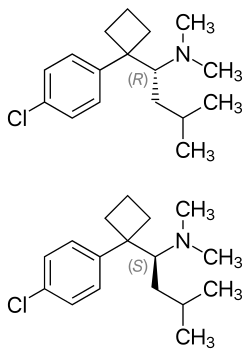
|
|||||||||||||
| ( R ) -form (top) and ( S ) -form (bottom), 1: 1 stereoisomeric mixture | |||||||||||||
| General | |||||||||||||
| Non-proprietary name | Sibutramine | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 17 H 26 ClN | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| ATC code | |||||||||||||
| Drug class | |||||||||||||
| properties | |||||||||||||
| Molar mass | |||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
193.6 ° C (racemic hydrochloride) |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Sibutramine is a medicinal substance that is used as an appetite suppressant ( anorectic ) to reduce excessive weight ( obesity ). The manufacturer of the original preparation was the German company Knoll AG, which now belongs to the pharmaceutical company Abbott Laboratories in the USA. The effect comes about through the indirect stimulation of the sympathetic nervous system . Drugs containing sibutramine have now been withdrawn from the market in all industrialized countries due to severe side effects.
chemistry
Sibutramine is an amphetamine - derivative . Which is used as a drug hydrochloride - monohydrate of sibutramine.
synthesis
The free base of sibutramine can be produced in a four-step synthesis. In the first reaction step , starting from (4-chlorophenyl) acetonitrile, the cyclobutane structure is built up by reaction with 1,3-dibromopropane using sodium hydride . The addition of 2-methylpropylmagnesium bromide to the nitrile function results in an azomethine structure in the second step, which is first converted into a primary amine by reduction with sodium borohydride . The N -alkylation takes place in the fourth reaction step using formaldehyde and formic acid . The synthesis sequence gives racemic sibutramine base. The hydrochloride monohydrate is formed during precipitation with hydrochloric acid .
Stereoisomerism
Sibutramine has a stereogenic center. So there are two enantiomers , the ( S ) -form and ( R ) -form. The racemate (1: 1 mixture of the enantiomers) is used for medicinal purposes, although for fundamental considerations it would be preferable to use the enantiomer which is more effective or has fewer side effects.
pharmacology
Mechanism of action
Sibutramine is an indirect sympathomimetic and can be administered orally. It inhibits the re-uptake of the neurotransmitters noradrenaline and serotonin in the nerve cells , which increases their concentration in the synaptic gap , which in turn increases the excitation of adrenoceptors and thus leads to a decrease in appetite.
Side effects
The side effects of sibutramine are significant and also the reason why the use of the active ingredient is severely restricted. They include numerous complaints from headache, dry mouth, nausea and vomiting to numbness, high blood pressure and cardiac arrhythmias .
The use of sibutramine is contraindicated for hyperthyroidism, angina pectoris , sleep disorders, high blood pressure, epilepsy , cardiac arrhythmias and disorders of liver and kidney function or when taking antidepressants and neuroleptics at the same time .
According to studies, sibutramine has a significantly increased risk of heart attack, especially for cardiovascular patients; a US study lists 34 deaths from the drug.
A 3-year study with patients who had both obesity and coronary artery disease at the same time achieved a weight reduction of around 3 kilograms on average. The study showed no survival benefit from sibutramine and an increased risk of cardiovascular events.
Risk-benefit assessment and impact on marketing
In Italy, medicines containing sibutramine were withdrawn from the market in 2002 due to two deaths. To investigate cardiovascular safety and the potential benefits of long-term use, a study called the Sibutramin Cardiovascular OUTcomes Trial (SCOUT) was conducted in 16 countries with approximately 10,000 subjects who took sibutramine for up to six years. Based on the results of the study, the Committee for Medicinal Products for Human Use of the European Medicines Agency assessed the risk-benefit ratio as negative in January 2010 and immediately recommended that the authorization for medicinal products containing sibutramine be suspended. The manufacturer of the original preparation then suspended marketing in the EU. In Germany, the suspension came into effect immediately, as only pharmaceuticals from the original manufacturer were on the market. Medicinal products containing sibutramine were previously only approved as medicinal products in very limited doses in Germany . In March 2010, the authorities in Poland ordered a suspension of the approval of all preparations, as was the case in Switzerland.
Due to the unfavorable risk-benefit ratio found in the SCOUT study, Abbott, in coordination with the FDA, also withdrew the drug from the US market in October 2010.
Trade names
Monopreparations :
Reductil , Reduxade , Zelium (D, A, CH; except for trade), Meridia (USA, except for trade), LiDa (China)
Prohibited trade and use
Sibutramine-containing products are illegally marketed to reduce body weight, to increase physical performance and to increase the effectiveness of coffee.
While 125,000 illegally imported capsules of the Chinese drug “ LiDa ” were confiscated in Germany in 2005 , this number exceeded the million mark in 2006. In 2011, customs authorities destroyed 50,000 slimming pills containing sibutramine that were seized at Frankfurt Airport and made in China.
The Hessian Ministry of Consumer Protection warned on December 2, 2011 against the consumption of certain batches of the coffee " Slimming Coffee Leisure 18 ", which is also offered on the Internet . Two batches of the “ Vitaccino ” brand instant coffee , in which sibutramine was detectable, were withdrawn from the market in 2010; a warning was given against consuming this product in 2013; customs had previously secured deliveries of goods ordered online because of the same ingredient.
In 2006, Sibutramine was included by the World Anti-Doping Agency (WADA) as a prohibited active ingredient in group S6, stimulants.
Individual evidence
- ↑ PR Oliveira, HK Stulzer, LS Bernardi, SHM Borgmann, SG Cardoso, MAS Silva: Sibutramine hydrochloride monohydrate - thermal behavior, decomposition kinetics and compatibility studies in J. Therm. Anal. Calorim. 100 (2010) 277-282, doi: 10.1007 / s10973-009-0200-7 .
- ↑ a b Datasheet Sibutramine hydrochloride monohydrate, ≥98% (HPLC), solid from Sigma-Aldrich , accessed on December 21, 2019 ( PDF ).
- ↑ a b c A. Kleemann , J. Engel, B. Kutscher D. Reichert: Pharmaceutical Substances - Synthesis, Patents, Applications , 4th edition (2000), Thieme-Verlag Stuttgart, pp. 1872–1873, ISBN 978-1 -58890-031-9 .
- ↑ Donald Eugene Butler, Joy C. Pollatz: Facile cycloalkylation of arylacetonitriles in dimethyl sulfoxide . In: J. Org. Chem. Band 36 , no. 9 May 1971, p. 1308-1309 , doi : 10.1021 / jo00808a035 .
- ↑ EJ Ariëns: Stereochemistry, a basis for sophisticated nonsense in pharmacokinetics and clinical pharmacology , European Journal of Clinical Pharmacology 26 (1984) 663-668. doi: 10.1007 / BF00541922 .
- ↑ Lüllmann, Mohr, Hein: Pocket Atlas of Pharmacology (5th edition). P. 95.
- ↑ a b Information about the drug Reduktil ( Memento from January 1, 2006 in the Internet Archive ) . On: www.adipositas-online.de from 2005.
- Jump up ↑ W. Philip T. James, Ian D. Caterson, Walmir Coutinho, Nick Finer, Luc F. Van Gaal, Aldo P. Maggioni, Christian Torp-Pedersen, Arya M. Sharma, Gillian M. Shepherd, Richard A. Rode, Cheryl L. Renz: Effect of Sibutramine on Cardiovascular Outcomes in Overweight and Obese Subjects. In: The New England Journal of Medicine . 2010, 363, 10, pp. 905-917, doi: 10.1056 / NEJMoa1003114 .
- ↑ Sibutramine appetite suppressant: demand for market withdrawal . Deutsches Ärzteblatt 99, issue 14 of April 5, 2002, page A-897 / B-749 / C-701.
- ↑ Sibutramine-containing medicinal products: approval has been suspended due to an increased risk of cardiovascular events. BfArM , January 25, 2010, accessed May 3, 2017 .
- ↑ The marketing authorization for Reductil is suspended due to the observation of an increased cardiovascular risk in the context of the SCOUT study (PDF; 334 kB) Rote Hand Brief dated January 22, 2010.
- ↑ a b Federal Ministry of Finance: Slimming pills with life-threatening side effects ( Memento from February 10, 2007 in the Internet Archive ) . On: Customs online on June 2, 2006.
- ↑ suspension of Meridia, Abbott (PDF file, 595 kB).
- ↑ suspension of Obesan, Sandoz (PDF file, 603 kB).
- ↑ Suspension of Sibutramine 1A, company 1A Pharma (PDF file; 615 kB).
- ↑ suspension of Afibron, company Teva (PDF file, 585 kB).
- ↑ Suspension of Zelixa, company Biofarm (PDF file; 602 kB).
- ↑ Swissmedic suspends approval of the appetite suppressant Reductil in Switzerland ( memento of June 5, 2011 in the Internet Archive ), Swissmedic of March 29, 2010, accessed on March 31, 2010.
- ^ Official notice of the FDA dated October 8, 2010 , accessed October 12, 2010
- ↑ Customs investigation destroys drugs and doping agents as well as counterfeit branded textiles ( memento of October 12, 2013 in the web archive archive.today ), Frankfurt am Main customs investigation office, August 17, 2011
- ↑ www.hmuelv.hessen.de: Warning of Slimming Coffee Leisure 18 ( Memento from January 23, 2016 in the Internet Archive ) ( MS Word ; 115 kB).
- ↑ Pharmaceutical agents in instant coffee. Imperia Elita calls back "Vitaccino" , Frankfurter Rundschau, October 7, 2010
- ↑ Sibutramine in coffee: Company recalls instant product ( Memento from October 14, 2013 in the Internet Archive ) Deutsche Ärztezeitung, December 10, 2010
- ↑ Prohibited active ingredient: Warning against slimming coffee "Vitaccino" ( Memento from June 2, 2015 in the Internet Archive ), State Investigation Office Rhineland-Palatinate, Koblenz March 4, 2013.
- ^ The World Anti-Doping Code - The 2006 Prohibited List .
