Multi-enzyme complex
Multi-enzyme complexes are groups of enzymes that catalyze successive steps in the cell's metabolism and are associated with one another . All organisms use this principle, which is an important step in improving catalytic efficiency . The advantages are obvious:
- enzymatic conversions are limited by diffusion processes ; H. by the frequency with which enzymes hit their substrates. In multi-enzyme complexes, the distance that a reaction product (that is the substrate of the subsequent reaction) has to travel is minimized, since the reaction intermediates remain bound to the complex;
- where applicable, the hydration of the product and the stripping of the hydrate shell before the next enzymatic conversion are omitted ;
- the principle reduces the chance that metabolites will undergo inappropriate side reactions ;
- Multi-enzyme complexes can be subjected to uniform control mechanisms.
Examples
The pyruvate dehydrogenase complex
This complex is the gateway for pyruvate to enter the citric acid cycle of the mitochondria . In its entirety, it is regulated by its own reaction product, acetyl-coenzyme A (AcCoA). It contains several units of three enzymes
- E1 pyruvate dehydrogenase ( EC 1.2.4.1 )
- E2 dihydrolipoyl transacetylase ( EC 2.3.1.12 )
- E3 dihydrolipoyl dehydrogenase ( EC 1.8.1.4 )
The "glycolysis complex"
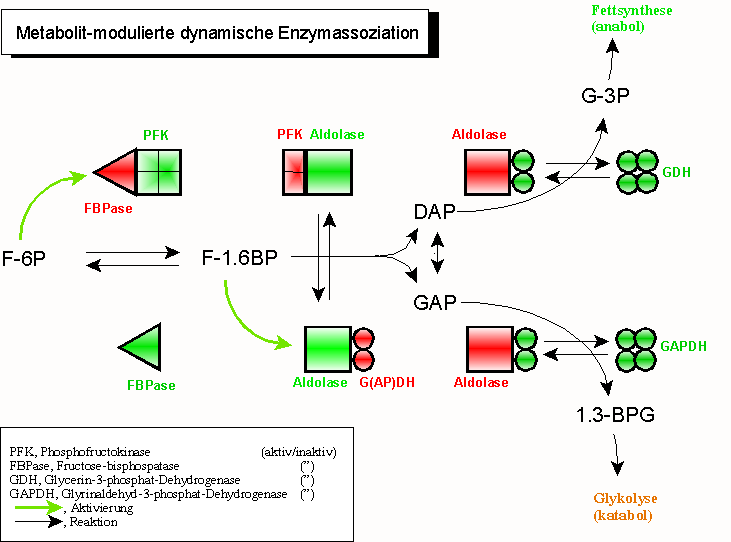
Another perfection of this principle was discovered several years ago by his pioneer, Sidney A. Bernhard (1927–1988; University of Oregon, Eugene), but has largely remained unnoticed: the “metabolite-modulated dynamic enzyme association” of successive members of a reaction chain. Here, according to the principle of a dynamic association, heterologous enzyme pairings are initiated, which last until a substrate is converted. After this event, the " donor enzyme " dissociates while the " acceptor enzyme " associates. In a constant change of acceptor and donor functions, metabolites are "passed on" and changed.
This principle explains the fact that successive enzymes such as glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and phosphoglycerate kinase (PGK) of glycolysis are difficult to separate from each other - and if so, then with a loss of activity.
Steps in detail
-
Fructose-1,6-bisphosphatase (FBPase, red) is kept in check by active phosphofructokinase (PFK, green). This prevents the simultaneous occurrence of opposing reactions in most (but not all) tissues;
- Simultaneous activity of PFK and FBPase in a so-called " substrate cycle " ( "futile cycle" ) can, however, be useful (regulatory phenomenon, heat generation in some insects);
- Fructose-1.6-bisphosphate (F-1.6BP) binds to aldolase and thereby causes
- the dissociation of the now inactive (red) precursor enzyme PFK; at the same time it causes the association of the still inactive secondary enzyme (glyceraldehyde-3-phosphate dehydrogenase, GAPDH, or glycerol phosphate dehydrogenase, GDH)
- Entering the GDH (NADH, H + excess) or GAPDH pathway (NAD + excess) is determined by the energy status of the cell (NADH, H + deficiency activates energy-generating, catabolic pathways, here glycolysis). The activation of GDH and GAPDH is supported by the association of the inactive (red) dimer forms into the active tetramers (green).
literature
- DK Srivastava, SA Bernhard: Metabolite transfer via enzyme-enzyme complexes. In: Science , Vol. 234, 1986, pp. 1081-1086.
- MF Dunn, GL Rossi: Obituary: Recollections of Sidney Bernhard (1927–1988). In: Trends Biochem. Sci. , Vol. 15, 1990, pp. 84-85.
- Eckhart Schweizer: Multi-enzyme complexes . In: Chemistry in Our Time . tape 7 , no. 1 , 1973, p. 25-31 , doi : 10.1002 / ciuz.19730070105 .
Individual evidence
- ↑ Judit Ovádi: Old pathway - new concept: control of glycolysis by metabolite- modulated dynamic enzyme associations . In: Trends in Biochemical Sciences . tape 13 , no. 12 , p. 486-490 , doi : 10.1016 / 0968-0004 (88) 90237-x ( cell.com [PDF; accessed October 15, 2017]).