Xanthophylls
| Structures of some xanthophylls |
|---|
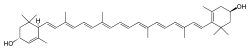 Lutein (CAS number: 127-40-2) |
|
Zeaxanthin (CAS number: 144-68-3) |
 Astaxanthin (CAS number: 472-61-7) |
 Canthaxanthin (CAS number: 514-78-3) |
 Capsanthin (CAS number: 465-42-9) |
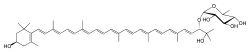 Myxoxanthophyll (CAS number: 11004-68-5) |
 Fucoxanthin (CAS number: 3351-86-8) |
|
Sarcinaxanthin (CAS number: 11031-47-3) |
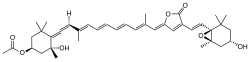 Peridinin (CAS number: 33281-81-1) |
 Dinoxanthin (CAS number: 54369-12-9) |
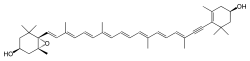 Diadinoxanthin (CAS number: 18457-54-0) |
 Staphyloxanthin (CAS number: 71869-01-7) |
Xanthophylls ( ancient Greek ξανθός xanthós "yellow", φύλλον phyllon "leaf") are the second important group of carotenoids next to the carotenes .
Similar to carotenes, xanthophylls are mostly tetraterpenes , but they have oxygen-containing groups in the form of hydroxyl (-OH), carbonyl (-C = O) and carboxy groups (-COOH) and, more rarely, oxirane groups .
Occurrence and function
Xanthophylls occur in the plastids of all photosynthetically active cells of plants . In the domain of the eukaryotes , they can only be synthesized by plants; the representatives found in animals are supplied through the diet.
Xanthophylls are also found in green-colored leafy vegetables such as spinach (10 mg / 100 g), kale (20 mg / 100 g) and lettuce . In the domain of the prokaryotes , they are made by some bacteria that live in locations that are heavily exposed to light. As pigments in the cell membrane , they protect against photo-oxidation of cell components.
properties
Despite the polar groups, the xanthophylls are lipophilic and therefore often sparingly soluble; partly the solubility in water is improved by glycosylation with a monosaccharide . Xanthophylls are sensitive to heat; 60–100% of them are destroyed by cooking. They occur as dyes both in animals and in plants. Most xanthophylls are yellow , red or orange in color; blue or purple tones are less common . Xanthophylls can be oxidized with oxygen, e.g. B. to Mutatochrome .
Important representatives
- Astaxanthin (red-violet), main carotenoid of the marine fauna (e.g. salmon , lobster )
- Canthaxanthin (red), the coloring agent in crabs , flamingo feathers and chanterelles
- Capsanthin (red) and capsorubin (red), coloring agents of the pepper fruits
- Cryptoxanthin (red), dye in z. B. Sunflowers and Physalis
- Fucoxanthin (brown), the color of brown algae (Phaeophyceae)
- Lutein (yellow-orange), a dye in the chloroplasts found
- Myxoxanthophyll (purple), a dye of the cyanobacteria
- Sarcinaxanthin (yellow), a dye found in certain types of bacteria (e.g. Micrococcus luteus )
- Staphyloxanthin (golden yellow), a dye found in the bacterium Staphylococcus aureus .
- Violaxanthin (yellow), a leaf pigment
- Zeaxanthin (orange-yellow), most important protective pigment of the photosynthetic apparatus ( xanthophyll cycle )
use
Xanthophylls are added to animal feed to produce color in animal products. For example, astaxanthin is added to chicken feed to intensify the color of the egg yolk and added to fishmeal for farmed salmon to give the meat a reddish color. For direct dyeing of cod-like Kohler , known as pollock , often canthaxanthin used. Lutein is used as a color in non-carbonated beverages, energy bars, and diet foods.
Individual evidence
- ↑ Wissenschaft-Online-Lexika: Entry on xanthophylls in the Lexikon der Biochemie , accessed on March 7, 2009.
- ↑ a b Science Online Lexica: Entry on xanthophylls in the Lexicon of Nutrition , accessed on March 7, 2009.
- ^ Hans G. Schlegel, Christiane Zaborosch: General microbiology . 7th edition. Thieme Verlag, Stuttgart / New York 1992, ISBN 3-13-444607-3 .
- ↑ R. Netzer, MH Stafsnes a. a .: Biosynthetic pathway for γ-cyclic sarcinaxanthin in Micrococcus luteus: heterologous expression and evidence for diverse and multiple catalytic functions of C (50) carotenoid cyclases. In: Journal of bacteriology. Volume 192, Number 21, November 2010, pp. 5688-5699, doi : 10.1128 / JB.00724-10 . PMID 20802040 . PMC 2953688 (free full text).
- ↑ Alexandra Pelz, Karsten-Peter Wieland, Karsten Putzbach, Petra Hentschel, Klaus Albert, Friedrich Götz: Structure and Biosynthesis of Staphyloxanthin from Staphylococcus aureus . In: Journal of Biological Chemistry , 280, 37, September 16, 2005, pp. 32493-32498, doi : 10.1074 / jbc.M505070200 .
- ↑ Textbook food chemistry and nutrition - By Robert Ebermann, Ibrahim Elmadfa, Springer Verlag ISBN 9783211486498 Google Books .
