Xanthophyll cycle
| Parent |
| Metabolism of the xanthophylls |
| Gene Ontology |
|---|
| QuickGO |
The xanthophyll cycle , also called violaxanthin cycle , is a protective mechanism of the photosynthetic apparatus of higher plants and some green algae . It helps to dissipate excess excitation energy as heat before reactive oxygen species form. This minimizes damage to the antenna complexes of the photosystem II (PS II). The xanthophyll cycle is an important step in the non-photochemical quenching of exciton energy.
This can occur if the electron transport chain is over-energized, for example due to excessive lighting (strong light stress).
Non-photochemical quenching of light energy
Photosynthesis uses the energy of light. The photosynthesis rate depends on the light intensity, but is limited to a certain extent. At high light intensities, many chlorophyll molecules are therefore excited in the antenna complexes, but due to the exhausted photosynthesis capacity they cannot pass their light energy on to the reaction center of photosynthetic system II (PS II). This has the consequence that the excited chlorophyll molecules generate reactive oxygen species (ROS), for example singlet oxygen 1 O 2 . ROS damage pigments , proteins and lipids of the thylakoid membrane and thereby inhibit photosynthesis or destroy photosynthetic systems.
To trap excess energy, plants use the xanthophyll cycle as a protective mechanism.
biochemistry
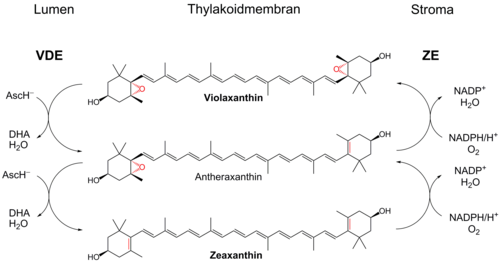
Violaxanthin deepoxidase (VDE)
In intense light, many protons are translocated into the thylakoid lumen as a result of efficient photosynthesis . These are normally used to drive a membrane-bound ATP synthase . At high light intensities, however, this proton gradient is not broken down quickly enough because the plant's ATP needs are met. As a result, the lumen becomes strongly acidic ( pH decrease). This activates the enzyme violaxanthin-Deepoxidase ( EC 1.10.99.3 ), which is the di epoxide violaxanthin over the monoepoxide antheraxanthin to zeaxanthin reduced. VDE is a nucleus-encoded enzyme which is localized in the lumen of the chloroplast and has a pH optimum of 5.0. The enzyme is inactive in the dark and is not associated with the membrane.
Ascorbate (AscH - ), which also activates the enzyme, is required for the reduction . This is oxidized to dehydroascorbate (DHA), which also releases water. DHA cannot be regenerated to ascorbate in the lumen. In addition, AscH - as an anion, cannot simply diffuse through the thylakoid membrane. There may be a transporter system that transports ascorbate into and DHA out of the lumen. In the stroma of the chloroplasts, DHA can be reduced to ascorbate by consuming NADPH and glutathione .
Zeaxanthin Epoxidase (ZE)
The reverse reaction of zeaxanthin via antheraxanthin to violaxanthin is catalyzed by another membrane-associated enzyme, zeaxanthin epoxidase ( EC 1.14.13.90 ). It is located in the stroma and introduces an epoxide group each with the consumption of oxygen and NADPH. The pH optimum for this monooxygenase is 7.5. The reaction takes place in plants in the dark or weak light, but has also been observed for bright light. Further cofactors such as FAD and ferredoxin are required for the reaction.
VDE and ZE are plant-based lipocalin proteins and share structural similarities.
meaning
Plants have many options for protecting the photosystem, but carotenoids are the most important protection systems. Here, the xanthophyll cycle as a non-constitutive protective mechanism against an oversupply of light energy is of the greatest importance. In the light- harvesting complexes , zeaxanthin binds to a subunit of LHCII that is protonated below the low pH. This effectively enables the absorption of the energy from chlorophyll in the triplet state ( 3 Chl a * ), which is then radiated as heat. This is because stimulated zeaxanthin only has a short lifespan of 10 ps. Violaxanthin, on the other hand, transmits excitation energy to chlorophyll and thus functions as an accessory pigment. A high level of excitation of the PS II leads to a high pH gradient; only then can zeaxanthin be formed. It is estimated that 50 to 70% of all photons absorbed are converted into heat by the cycle.
Diadinoxanthine cycle
In diatoms , a similar cycle is used for non-photochemical quenching, in which diatoxanthin (Dtx) is used instead of zeaxanthin. This is converted by the Dtx-Epoixdase (DEP) to diadionoxanthin (Ddx) with consumption of NADPH and O 2 . Diadionoxanthin is therefore functionally equivalent to violaxanthin. Starting from Dtx, Ddx is recycled by the diadinoxanthin deepoxidase (DDE).
In this cycle there is no connection with a diepoxide, and the conversion of Dtx to Ddx is very fast.
Diadinoxanthine deepoxidase (DDE)
The enzyme DDE has an optimum pH of 5.5, but also shows activity in a neutral environment. It requires ascorbate (AscH - ) as a cofactor to reduce the epoxide. As with violaxanthin deepoxidase (VDE), water is also released. In contrast to the VDE, DDE shows some differences: DDE requires less ascorbate and its activity is stimulated by a lower concentration of monogalactosyldiacylglycerol.
Diatoxanthin Epoxidase (DEP)
For the oxidation of diatoxanthin to diadionoxanthin, DEP requires the same cofactors as the zeaxanthin epoxidase (ZE): NAD (P) H, FAD, ferredoxin and oxygen. This catalyzed reaction takes place at an optimum pH of 7.5. In the dark and with a strong (light-driven) pH gradient on the membrane, DEP is completely inhibited. There is probably not enough NADPH available during the dark.
meaning
For diatoms, the diadinoxanthine cycle is the most important protective mechanism in the event of excessive stimulation of the photosynthetic apparatus. In contrast to the xanthophyll cycle, the reactions are much faster.
Lutein epoxy cycle
In some plant species, the lutein-epoxide cycle serves as a protective mechanism. In this process, lutein-epoxide is alternately converted into lutein, with the xanthophyll cycle also running in parallel with this cycle. The cycle occurs in plants that grow in the deep undergrowth of forests.
Individual evidence
- ↑ a b c Hans W. Heldt and Birgit Piechulla: Plant biochemistry . Spectrum Akademischer Verlag GmbH, 4th edition 2008; ISBN 978-3-8274-1961-3 ; Pp. 109-110.
- ↑ a b Szabó, I. et al . (2005): Light and oxygenic photosynthesis: energy dissipation as a protection mechanism against photo-oxidation . In: EMBO Rep. 6 (7); 629-634; PMID 15995679 ; PMC 1369118 (free full text)
- ^ Hieber, AD. et al . (2000): Plant lipocalins: violaxanthin de-epoxidase and zeaxanthin epoxidase . In: Biochim Biophys Acta 1482 (1-2); 84-91; PMID 11058750 ; doi: 10.1016 / S0167-4838 (00) 00141-2 .
- ↑ a b Jahns, P. et al . (2009): Mechanism and regulation of the violaxanthin cycle: the role of antenna proteins and membrane lipids . In: Biochim Biophys Acta 1787 (1); 3-14; PMID 18976630 ; doi: 10.1016 / j.bbabio.2008.09.013 .
- ↑ Caroline Bowsher, Martin Steer and Alyson Tobin: Plant Biochemistry . Garland Pub 2008; ISBN 978-0-8153-4121-5 ; P. 90.
- ↑ a b c d Wilhelm, C. et al . (2006): The regulation of carbon and nutrient assimilation in diatoms is significantly different from green algae . In: Protist. 157 (2); 91-124; PMID 16621693 ; doi: 10.1016 / j.protis.2006.02.003 .
- ↑ Grouneva, I. et al . (2009): The regulation of xanthophyll cycle activity and of non-photochemical fluorescence quenching by two alternative electron flows in the diatoms Phaeodactylum tricornutum and Cyclotella meneghiniana . In: Biochim Biophys Acta 1787 (7); 929-938; PMID 19232316 ; doi: 10.1016 / j.bbabio.2009.02.004 .
- ↑ García-Plazaola, JI. et al . (2007): The lutein epoxide cycle in higher plants: its relationships to other xanthophyll cycles and possible functions . In: Functional Plant Biology 34 (9); 759-773; doi: 10.1071 / FP07095 .
- ↑ Bungard, RA. et al . (1999): Unusual carotenoid composition and a new type of xanthophyll cycle in plants . In: Proc Natl Acad Sci USA 96 (3); 1135-1139; PMID 9927706 ; PMC 15363 (free full text)
literature
- Jahns, P. et al . (2009): Mechanism and regulation of the violaxanthin cycle: the role of antenna proteins and membrane lipids . In: Biochim Biophys Acta 1787 (1); 3-14; PMID 18976630 ; doi: 10.1016 / j.bbabio.2008.09.013
- García-Plazaola JI1, Esteban R, Fernández-Marín B, Kranner I, Porcar-Castell A. (2012): Thermal energy dissipation and xanthophyll cycles beyond the Arabidopsis model . Photosynth Res. 113 (1-3): 89-103. doi: 10.1007 / s11120-012-9760-7 ; PMID 22772904
