Dehydroascorbic acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
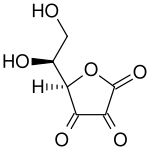
| L -isomer of dehydroascorbic acid | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Dehydroascorbic acid | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 6 H 6 O 6 | |||||||||||||||||||||
| Brief description |
beige solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 174.11 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Dehydroascorbic acid (English dehydro ascorbic acid, DHA ) is a chemical compound from the group of ascorbic acid derivatives .
Occurrence
L -Dehydroascorbic acid is produced by the oxidation of ascorbic acid . In the human metabolism , it can be reduced to L -ascorbic acid and thus regenerate vitamin C. The oxidation of ascorbic acid to dehydroascorbic acid takes place in many tissues, whereas the reconversion of dehydroascorbic acid to ascorbic acid seems to take place primarily in the liver.
In general, vitamin C in the form of DHA is transported into the mitochondria of the cells by glucose transporters, mainly GLUT-1 , as only very few cells have specific vitamin C transporters. Most of these transporters are sodium ion-dependent. The brain in particular is dependent on a supply of ascorbic acid, but the vitamin cannot cross the blood-brain barrier . This problem is circumvented by the fact that dehydroascorbic acid is transported through the barrier by glucose transporters, for example GLUT1, and is reduced to ascorbic acid in the brain cells.
It is assumed that ascorbic acid is transported intracellularly in the form of DHA. Here, extracellular ascorbic acid should be oxidized to DHA, absorbed into the cell and then reduced again, since ascorbic acid itself cannot leave the cell. DHA is more unstable than L -ascorbic acid. Depending on the reaction conditions (pH, presence or absence of reducing agents such as glutathione ), it can either be converted back into ascorbic acid or irreversibly hydrolyzed to diketogulonic acid (DKG).
Extraction and presentation
Pure dehydroascorbic acid can be obtained by oxidizing ascorbic acid with oxygen in methanol with the aid of a catalyst.
properties
L -Dehydroascorbic acid is a beige solid. It exists in the solid state as a dimer and becomes a monomer in solution. In aqueous solutions, dehydroascorbic acid is almost completely present as a monohydrate (mono-DHA · H 2 O). It forms a bicycle, which has been proven by nuclear magnetic resonance spectroscopy . It may be able to absorb a second molecule of water in order to then form a dihydrate. Semi-dehydroascorbic acid and oxidized forms of esterified ascorbic acids are also included in the group of dehydroascorbic acid.
Individual evidence
- ↑ a b c d e f data sheet (L) -Dehydroascorbic acid from Sigma-Aldrich , accessed on May 4, 2017 ( PDF ).
- ↑ Peter M. Collins: Dictionary of Carbohydrates . CRC Press, 2005, ISBN 978-0-8493-7765-5 , pp. 281 ( limited preview in Google Book search).
- ↑ Hartmut Dunkelberg, Thomas Gebel, Andrea Hartwig: Vitamins and trace elements requirements, deficiencies, hypervitaminoses and food supplements . John Wiley & Sons, 2013, ISBN 978-3-527-65307-2 , pp. 78 ( limited preview in Google Book search).
- ↑ Lohmann: The metabolism, second part . Springer-Verlag, 2013, ISBN 978-3-662-21764-1 , pp. 383 ( limited preview in Google Book search).
- ↑ KC Sagun et al: Vitamin C enters mitochondria via facilitative glucose transporter 1 (Glut1) and confers mitochondrial protection against oxidative injury. In: FASEB Journal . 2005, 19 (12), pp. 1657-1667, PMID 16195374 ( PDF , free full text access ).
- ↑ J. Huang et al: Dehydroascorbic acid, a blood-brain barrier transportable form of vitamin C, mediates potent cerebroprotection in experimental stroke. In: PNAS . 2001, 98, No. 20, pp. 11720-11724, PMID 11573006 ( PDF , free full-text access ).
- ↑ CS Tsao: An overview of ascorbic acid chemistry and biochemistry. In: Lester Packer, Jürgen Fuchs: Vitamin C in Health and Disease. Marcel Dekker Inc illustrated edition 1997, ISBN 0-8247-9313-7 , pp. 25-58.
- ^ Y. Nishikawa and T. Kurata: Interconversion between dehydro-L-ascorbic acid and L-ascorbic acid. In: Bioscience, Biotechnology, and Biochemistry . 2000, 64 No. 3, pp. 476–483, PMID 10803943 , doi: 10.1271 / bbb.64.476 , PDF ( memento from December 19, 2014 in the Internet Archive ), free full-text access .
- ↑ Y. Nishikawa et al. a: Identification of 3,4-dihydroxy-2-oxo-butanal (L-threosone) as an intermediate compound in oxidative degradation of dehydro-L-ascorbic acid and 2,3-diketo-L-gulonic acid in a deuterium oxide phosphate buffer. In: Bioscience, Biotechnology, and Biochemistry . 2001, 65, No. 8, pp. 1707–1712, PMID 11577707 , doi: 10.1271 / bbb.65.1707 , PDF ( memento from December 19, 2014 in the Internet Archive ), free full-text access .
- ^ Wilhelm Friedrich: Vitamins . Walter de Gruyter, 1988, ISBN 978-3-11-010244-4 , p. 936 ( limited preview in Google Book search).
- ^ W. Bors and GR Buettner: The vitamin C radical and its reactions. In: Lester Packer, Jürgen Fuchs: Vitamin C in Health and Disease. Marcel Dekker Inc illustrated edition 1997, ISBN 0-8247-9313-7 , p. 76.