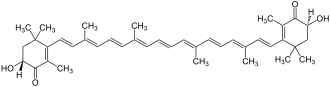
Astaxanthin
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
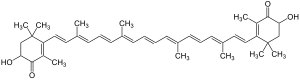
| Astaxanthin without a precise configuration of the alcohols | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Astaxanthin | |||||||||||||||||||||
| other names |
3,3'-dihydroxy-β, β-carotene-4,4'-dione |
|||||||||||||||||||||
| Molecular formula | C 40 H 52 O 4 | |||||||||||||||||||||
| Brief description |
purple plates |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 596.85 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
215–216 ° C (decomposition) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Astaxanthin ( AX , from the Greek. Ἀστακός ( Astakós ) "Hummer" and xanthine to Greek. Ξανθός ( Xanthos ) "yellow") is a natural, reddish-purple dye that for xanthophyll class of carotenoids counts. It is mainly produced by green algae and is responsible, for example, for the red color of crustaceans that consume these algae. Astaxanthin is obtained industrially from the alga Haematococcus pluvialis , which is also responsible for the natural phenomenon of "blood rain" . The dye was formerly known as a hematochrome .
Properties and use

Astaxanthin is structurally related to the carotenoids β-carotene , zeaxanthin, and lutein , and so they share many of the metabolic and physiological functions ascribed to the carotenoids.
In fish nutrition it is important that astaxanthin has a vitamin-like effect and thus has a positive effect on fertility and the immune defense of fish in breeding facilities. In fish, astaxanthin, which has a ten times stronger effect than β-carotene , not only intensifies the red, but also the yellow, green and blue pigments and makes the meat salmon-red. The natural color of the flesh of salmon is due to the astaxanthin content of the small crustaceans consumed.
Astaxanthin is approved as a feed additive (E 161j) for fish feed in the production of edible fish. It is used to feed the white-fleshed rainbow trout with salmon-red meat. They are then marketed as salmon trout .
Astaxanthin can protect the skin from the stress caused by UV rays due to its absorption and its properties as an antioxidant . It acts in this capacity considerably stronger than vitamin E . Ingested astaxanthin supplements the protective effects of sunscreens and is used by some Ironman athletes.
Stereoisomers
Astaxanthin contains a stereocenter in each of the cyclohexenone rings, so there are three isomeric astaxanthines:
| Astaxanthin stereoisomers |
|
(3 S , 3 ′ S ) -astaxanthin
|
|
(3 R , 3 ′ R ) -astaxanthin
|
|
meso- astaxanthin
|
- (3 S , 3 ′ S ) -astaxanthin,
- (3 R , 3 ′ R ) -astaxanthin and
- meso- astaxanthin.
Wild salmon contains almost exclusively (3 S , 3 ′ S ) -astaxanthin. If farmed salmon is fed synthetic astaxanthin, the stereoisomers (3 S , 3 ′ S ): meso : (3 R , 3 ′ R ) in a ratio of 1: 2: 1 are found in the fish . B. from Pandalus borealis ), the meso form dominates . If the astaxanthin comes from the yeast Xanthophyllomyces dendrohous , the (3 R , 3 ′ R ) stereoisomer is found in salmon .
Individual evidence
- ↑ Entry on astaxanthin. In: Römpp Online . Georg Thieme Verlag, accessed on August 20, 2014.
- ↑ Data sheet Astaxanthin from Acros, accessed on February 26, 2010.
- ↑ Data sheet Astaxanthin, ≥98% (HPLC) from Sigma-Aldrich , accessed on February 10, 2013 ( PDF ).
- ↑ Martin Guerin, Mark E. Huntley, Miguel Olaizola: Haematococcus astaxanthin: applications for human health and nutrition. In: Trends in Biotechnology . 21, No. 5, 2003, pp. 210-216, doi : 10.1016 / S0167-7799 (03) 00078-7 .
- ↑ Ghazi Hussein et al. a .: Astaxanthin, a carotenoid with potential in human health and nutrition. In: Journal of Natural Products . 69, No. 3, pp. 443-449, doi : 10.1021 / np050354 + .
- ↑ Jörg Zittlau : Skin cancer: tea and pills work better than sunscreen. In: Welt-Online from July 13, 2010.
- ↑ Bernd Schäfer: Natural substances in the chemical industry , Spektrum Akademischer Verlag, 2007, p. 426, ISBN 978-3-8274-1614-8 .