Staphyloxanthin
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
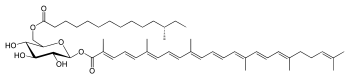
|
|||||||||||||
| General | |||||||||||||
| Surname | Staphyloxanthin | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 51 H 78 O 8 | ||||||||||||
| Brief description |
golden yellow dye |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 819.2 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||

Staphyloxanthin is an orange or golden yellow pigment in the cell membrane of the bacterium Staphylococcus aureus . The pigment belongs to the xanthophylls (oxygen-containing carotenoids ).
The carotenoid pigment acts as a biological antioxidant , by the by the immune cells of the host inactivates reactive oxygen species formed, and thereby the microorganisms protects against killing by the white blood cells.
Especially with the antibiotic- resistant super germs , the MRSA representatives, the spread of which has increased rapidly in the population and in hospitals since 1990, staphyloxanthin is an important virulence factor . At the same time, the dye protects the MRSA germs from being destroyed by hydrogen peroxide and oxygen radicals .
Occurrence
Staphyloxanthin is built into the cell membrane of the bacterium Staphylococcus aureus and is responsible for its coloring. The pigment is yellow or orange, depending on the oxygen supply and nutrient conditions. The color gives the species its name ( aureus , Latin for 'golden'). In addition to the main pigment, different colored intermediate products of the biosynthetic pathway can be isolated.
Layout and function
Structure elucidation
The pigment name, staphyloxanthin, was first mentioned in 1972 by John H. Marshall and ES Rodwell. In 1981 Marshall and Willmoth isolated and analyzed the chemical structures of the dye and other biosynthetic products. The main pigment was initially identified as an α- D -glucopyranosyl-1- O - (4,4ʹ-diaponeurosporen-4-oat) -6- O - (12-methyltetradecanoate), in which glucose with a triterpenoid carotenoid carboxylic acid and a fatty acid (C 15 ) is esterified .
In 2005, the structure as a β- D -glucopyranosyl-1- O- (4,4ʹ-diaponeurosporen-4-oat) -6- O- (12-methyltetradecanoate) compound could be determined by means of NMR spectroscopy . Staphyloxanthine differs from the carotenoids with 40 carbon atoms (C 40 ) that occur in photosynthetically and non-photosynthetically active bacteria and in eukaryotes in that glucose is esterified with a triterpenoid acid (C 30 ) and a fatty acid (C 15 ) and the hydroxyl groups of Glucose. The bacterial pigment is a representative of the triterpenes , which are among the secondary components of organisms. Formally, they are derived from isoprene . Staphylxanthine is a fat-soluble dye with long-chain hydrocarbons that belongs to the group of carotenoids. Carotenoids are commonly found in many photosynthetic and non-photosynthetic organisms and have antioxidant functions in bacteria and yeast. They play a role in light generation, energy transfer and the regulation of membrane fluidity.
Physiological importance
Numerous conjugated double bonds enable staphyloxanthin to absorb free energy from reactive oxygen compounds and render it harmless. As a radical scavenger , the carotenoid can quench singlet oxygen and protect the microbes from aggressive hydrogen peroxide and hydroxyl radicals . These compounds are particularly important in the immune response in the human body. Here in are phagosomes of neutrophils through the NADPH - Oxidase reactive oxygen species formed for the removal of pathogens. Staphylococcus aureus bacteria can survive this with the help of the antioxidant effect of staphyloxanthin.
As a membrane carotenoid, the dye influences the fluidity of the cell membrane and thus the membrane strength. This affects the chemical properties and the functional activity of the bacterial cell membrane. Staphylococcal bacteria pigmented with staphyloxantin therefore have a higher resistance because they have a better chance of survival against antimicrobial peptides and proteases produced by the host's cells . This means that colored staphylococci have a higher vitality than white representatives . In addition, the dye protects the microbes from photo-oxidative destruction.
Chemical-physical properties
Staphyloxanthin its molecular properties dissolves due to bad in polar solvents, however, well in chloroform and chloroform- methanol - mixtures . Purified staphyloxanthin dissolves very well in petroleum ether .
Isolated staphyloxanthine has an absorption maximum in visible light at a wavelength of λ = 460 nm in the solvent methanol or acetone and an absorption maximum of λ = 462 nm with a small side peak at λ = 491 nm in petroleum ether. HPLC-UV analyzes of purified staphyloxanthine using a linear acetone / water gradient showed an absorption spectrum with peaks at 463 nm and a side peak at 490 nm.
biosynthesis
The five structural genes for the synthesis of staphyloxanthine lie in the operon crtOPQMN ( CrtO , CrtP , CrtQ , CrtM and CrtN ). The AldH gene in Staphylococcus aureus , which is located 670 kilobase pairs beyond the operon, codes for a 4,4′-diaponeurospore aldehyde dehydrogenase ( AldH ). This enzyme is believed to be important for the complete synthesis of the dye. If this gene is missing, 4,4′-diaponeurospore-4-al accumulates and is not converted into staphyloxanthin.
Synthetic route
The synthesis of the triterpenes begins with the condensation of two molecules of farnesyl pyrophosphate (FPP) C 15 H 25 O 7 P 2 3− to 4,4′-diapophytoene (dehydrosqualene) C 30 H 48 , catalyzed by dehydrosqualene synthetase ( CrtM ). The yellow intermediate 4,4-diaponeurospores C 30 H 42 results from the subsequent oxidation by the dehydrosqualene desaturase CrtN . 4,4-Diaponeurosporen shows an absorption spectrum with three peaks in a petroleum-acetone mixture at the wavelengths of 412 nm, 435 nm and 465 nm. 4,4-Diaponeurosporen C 30 H 42 becomes the acid 4,4-Diaponeurospore acid C 30 H 40 O 2 oxidizes (gene CrtP ).
The glycosyltransferase CrtQ esterifies the hydroxyl group of glucose on the first carbon atom with 4,4-diaponeurospore acid. In the last biosynthesis step, the 12-methyltetradecanoic acid , a C 15 fatty acid, is linked to the hydroxyl group on the sixth carbon atom of glucose to form the golden-yellow pigment staphyloxanthine.
regulation
The staphyloxanthine biosynthesis is very complex and can be regulated at different metabolic levels. The operon in which the biosynthetic genes are organized is regulated by a sigma factor (B) -dependent promoter and terminated by a terminator sequence. The alternative sigma factor B, which binds to a specific DNA sequence in the crt promoter region of the operon, directly regulates the synthesis of staphyloxanthine. Deletion mutants that do not have sigma factor were white and showed no pigment formation.
In addition, the cold shock protein A (CspA) , which is dependent on the sigma factor, influences the operon and the synthesis of metabolic products as a positive regulator.
In addition to these mechanisms, other signal transduction systems act on the staphylococcal metabolism and on the staphyloxanthine synthesis.
Staphyloxanthine as a possible target in antimicrobial therapy
Staphylococcus aureus belongs to the normal colonization flora of humans and mainly colonizes the skin and mucous membranes. Staphylococcus aureus infections, such as mastitis , postoperative wound infections and boils , occuronly when there is an immunodeficiency caused by underlying human diseases. In addition to the staphyloxanthin, the Hauptpathogenitätsfaktor , this seed is a variety of other extracellular virulence factors such as hemolysin , coagulase , toxic shock syndrome and protein A . Food poisoning caused by enterotoxins caused by staphylococci is also feared.
Until the 1990s, staphylococci (MRSA) resistant to the antibiotic methicillin occurred only in hospitals. Thereafter, the spread of antibiotic-resistant infections also increased outside of hospitals; since 2005, MRSA infections have also been observed in farm animals. Due to the emergence of multidrug resistance strains , so-called super germs , these MRSA pathogens are also resistant to all other β-lactam antibiotics that were originally used to treat Staphylococcus aureus infections. The medical treatment of infections caused by microbes that are representative of linezolid -resistant MRSA ( LRSA ) and vancomycin -intermediate and -resistant MRSA ( VISA and VRSA ) requires the rapid development of new anti-infectives.
Treatment with Rhodomyrtus extract
Rhodomyrtus tomentosa (Aiton) Hassk. is an evergreen shrub plant of the genus Rhodomyrtus that the family of myrtle belongs. The plant is found in South and Southeast Asia and has been used in traditional Chinese and Malaysian medicine to treat various types of bacterial inflammation. In several studies in 2019, the scientists Thanh Sang Vo and Dai Hung Ngo were able to show the effect of the biologically active metabolites of Rhodomyrtus on various microorganisms.
In Staphylococcus aureus , an extract from Rhodomyrtus tomentosa inhibited staphyloxanthine biosynthesis and reduced resistance to hydrogen peroxide and singlet oxygen. In addition, disturbances of the bacterial cell wall biosynthesis and cell division as well as inhibitions of various enzymes of important metabolic pathways of Staphylococcus aureus could be detected, which reduced the vitality of these bacteria compared to those without treatment with the extract.
According to the studies, the active ingredients from Rhodomyrtus can be said to have a damaging effect on the bacterial cell membrane as well as a change in the fluidity of the cell membrane.
Exposure to blue light
In a study in 2019, Pu-Ting Dong et al. a. on biofilms of Staphylococcus aureus and on wound infections in mouse models show that staphyloxanthin was photochemically decomposed by exposure to blue light and thus lost its antioxidant effect. The orange pigmented MRSA germs showed a changed membrane permeability after irradiation in the visible light wavelength range of λ = 460 nm. This made the microbes more sensitive to hydrogen peroxide and other reactive oxygen compounds. The synergistic effect of photobleaching and weak disinfectants could be used promisingly for a new therapeutic treatment method to combat MRSA infections.
Web links
- Compound - C16148 in the Kyoto Encyclopedia of Genes and Genomes
- Entry CHEBI: 71690 in the ChEBI database of the European Molecular Biology Laboratory (EMBL)
Individual evidence
- ↑ a b c d e Alexandra Pelz, Karsten-Peter Wieland, Karsten Putzbach, Petra Hentschel, Klaus Albert, Friedrich Götz: Structure and Biosynthesis of Staphyloxanthin from Staphylococcus aureus . In: Journal of Biological Chemistry , 280, 37, September 16, 2005, pp. 32493-32498, doi : 10.1074 / jbc.M505070200 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b Pu ‐ Ting Dong, Haroon Mohammad, Jie Hui, Leon G. Leanse, Junjie Li, Lijia Liang, Tianhong Dai, Mohamed N. Seleem, Ji ‐ Xin Cheng: Photolysis of Staphyloxanthin in Methicillin ‐ Resistant Staphylococcus aureus Potentiates Killing by Reactive Oxygen Species . In: Advanced Science 6 (11), March 30, 2019, doi : 10.1002 / advs.201900030 , PMID 31179216 , PMC 6548961 (free full text).
- ↑ Chia-I Liu, George Y. Liu, Yongcheng Song, Fenglin Yin, Mary E. Hensler, Wen-Yin Jeng, Victor Nizet, Andrew H.-J. Wang, Eric Oldfield: A Cholesterol Biosynthesis Inhibitor Blocks Staphylococcus aureus Virulence . In: Science 319 (5868), March 2008, pp. 1391-1394, doi : 10.1126 / science.1153018 , PMC 2747771 (free full text).
- ^ Mary Barber: Pigment Production by Staphylococci . In: Microbiology 13, 2, October 1, 1955, pp. 338-345, doi : 10.1099 / 00221287-13-2-338 .
- ↑ Kiran B. Tiwari, Craig Gatto, Brian J. Wilkinson: Interrelationships among Fatty Acid Composition, Staphyloxanthin Content, Fluidity, and Carbon Flow in the Staphylococcus aureus Membrane . In: Molecules 2018, 23 (5), 1201, doi : 10.3390 / molecules23051201 .
- ↑ communicated in Alexandra Pelz, Karsten-Peter Wieland, Karsten Putzbach, Petra Hentschel, Klaus Albert, Friedrich Götz: Structure and Biosynthesis of Staphyloxanthin from Staphylococcus aureus . In: Journal of Biological Chemistry , 280, 37, September 16, 2005, pp. 32493-32498, doi : 10.1074 / jbc.M505070200 , PMID 16020541 .
- ↑ John H. Marshall, ES Rodwell in: 3rd International Symposium on Carotenoids Other Than Vitamin A, 4. – 7. September 1972 , pp. 56-57, International Union of Pure and Applied Chemistry , Cluj, Romania.
- ↑ John H. Marshall, Gregory J. Wilmoth: Pigments of Staphylococcus aureus, a Series of Triterpenoid Carotenoids . In: Journal of Bacteriology 147, 3, September 1981, pp. 900-913, PMID 7275936 , PMC 216126 (free full text) ,.
- ↑ Nancy E. Holt, Donatas Zigmantas, Leonas Valkunas, Xiao-Ping Li, Krishna K. Niyogi, Graham R. Fleming: Carotenoid Cation Formation and the Regulation of Photosynthetic Light Harvesting . In: Science 307, 5708, January 21, 2005, pp. 433-436, doi : 10.1126 / science.1105833 , PMID 15662017 .
- ↑ Ethan T. Johnson, Claudia Schmidt-Dannert: Light-energy conversion in engineered microorganisms . In: Trends in Biotechnology 26 (12), December 2008, pp. 682-689, doi : 10.1016 / j.tibtech.2008.09.002 . PMID 18951642 .
- ^ Henry Rosen, Seymor J. Klebanoff: Bactericidal activity of a superoxide anion-generating system. A model for the polymorphonuclear leukocyte . In: Journal of Experimental Medicine 149 (1), January 1979, pp. 27-39, doi : 10.1084 / jem.149.1.27 , PMID 216766 , PMC 2184741 (free full text).
- ↑ Thomas A. Dahl, W. Robert Midden, Philipe Hartman: Comparison of Killing of Gram-Negative and Gram-Positive Bacteria by Pure Singlet Oxygen . In: Journal of Bacteriology 171 (4), April 1989, pp. 2188-2194, doi : 10.1128 / jb.171.4.2188-2194.1989 , PMID 2703469 , PMC 209876 (free full text).
- ^ Norman I. Krinsky: Actions of Carotenoids in Biological Systems . In: Annual Review of Nutrition 13, July 1993, pp. 561-587, doi : 10.1146 / annurev.nu.13.070193.003021 , PMID 8369159 .
- ↑ Chia-I Liu, George Y. Liu, Yongcheng Song, Fenglin Yin, Mary E. Hensler, Wen-Yih Jeng, Victor Nizet, Andrew H.-J. Wang, Eric Oldfield: A Cholesterol Biosynthesis Inhibitor Blocks Staphylococcus aureus Virulence . In: Science 319 (5868), March 2008, pp. 1391-1394, doi : 10.1126 / science.1153018 , PMID 18276850 , PMC 2747771 (free full text).
- ↑ a b Alexandra Clauditz, Alexandra Resch, Karsten-Peter Wieland, Andreas Peschel, Friedrich Götz: Staphyloxanthin Plays a Role in the Fitness of Staphylococcus aureus and Its Ability To Cope with Oxidative Stress . In: Infection and Immunity 74 (8), August 2006, pp. 4950-4953, doi : 10.1128 / IAI.00204-06 , PMID 16861688 , PMC 1539600 (free full text).
- ↑ IA Popov, AS Kaprel'iants, DN Ostrovskii, VV Ignatov: [Study of the membranes of pigment-free mutant of Staphylococcus aureus] (Article in Russian). In: Biokhimiia 41 (6), July 1976, pp. 1116-1120, PMID 1027489 .
- ^ A b John H. Marshall, Gregory J. Wilmoth: Pigments of Staphylococcus aureus, a Series of Triterpenoid Carotenoids . In: Journal of Bacteriology 147, 3, September 1981, pp. 900-913, PMID 7275936 , PMC 216126 (free full text).
- ↑ a b Se Hyeuk Kim, Pyung Cheon Lee: Functional Expression and Extension of Staphylococcal Staphyloxanthin Biosynthetic Pathway in Escherichia coli . In: Journal of Biological Chemistry 287, 26, June 22, 2012, pp. 21575-21583, doi : 10.1074 / jbc.M112.343020 , PMID 22535955 , PMC 3381123 (free full text).
- ↑ Bernd Wieland, Corinna Feil, Eva Gloria-Maercker, Günther Thumm, Max Lechner, Jean-Michel Bravo, Karl Poralla, Friedrich Götz: Genetic and Biochemical Analyzes of the Biosynthesis of the Yellow Carotenoid 4,4'-Diaponeurosporene of Staphylococcus aureus . In: Journal of Biological Chemistry 176, December 24, 1994, pp. 7719-7726, doi : 10.1128 / jb.176.24.7719-7726.1994 , PMID 8002598 , PMC 197231 (free full text).
- ↑ a b Jeffrey W. Hall, Junshu Yang, Haiyong Guo, Yinduo Ji: The Staphylococcus aureus AirSR Two-Component System Mediates Reactive Oxygen Species Resistance via Transcriptional Regulation of Staphyloxanthin Production . In: Infection and Immunity 85 (2), January 26, 2017, doi : 10.1128 / IAI.00838-16 , PMID 27872240 , PMC 5278164 (free full text).
- ^ Ines Kullik, Philipp Giachino, Thomas Fuchs: Deletion of the Alternative Sigma Factor ςB in Staphylococcus aureus Reveals Its Function as a Global Regulator of Virulence Genes In: Journal of Biological Chemistry 180 (18), September 1998, pp. 4814-4820, PMID 9733682 , PMC 107504 (free full text).
- ↑ Samuel Katzif, Eun-Hee Lee, Anthony B. Law, Yih-Ling Tzeng, William M. Shafer: CspA Regulates Pigment Production in Staphylococcus aureus through a SigB-Dependent Mechanism . In: Journal of Bacteriology 187 (23), December 2005, pp. 8181-8184, doi : 10.1128 / JB.187.23.8181-8184.2005 , PMID 16291691 , PMC 1291268 (free full text).
- ↑ Meiying Yan, Jeffrey W. Hall, Junshu Yang, Yinduo Ji: The Essential yhcSR Two-Component Signal Transduction System Directly Regulates the lac and opuCABCD Operons of Staphylococcus aureus . In: PLoS One 7 (11), November 30, 2012, doi : 10.1371 / journal.pone.0050608 , PMID 23226327 , PMC 3511567 (free full text).
- ↑ Jeffrey W. Hall, Junshu Yang, Haiyong Guo, Yinduo Ji: The Staphylococcus aureus AirSR Two-Component System Mediates Reactive Oxygen Species Resistance via Transcriptional Regulation of Staphyloxanthin Production . In: Infection and Immunity 85 (2), January 26, 2017, doi : 10.1128 / IAI.00838-16 , PMID 27872240 , PMC 5278164 (free full text).
- ↑ Stien Vandendriessche, Wannes Vanderhaeghen, Filomena Valente Soares, Marie Hallin, Boudewijn Catry, Katleen Hermans, Patrick Butaye, Freddy Haesebrouck, Marc J. Struelens, Olivier Denis: Prevalence, risk factors and genetic diversity of methicillin-resistant Staphylococcus aureus carried by humans and animals across livestock production sectors . In: Journal of Antimicrobial Chemotherapy 68 (7), July 2013, pp. 1510-1516, doi : 10.1093 / jac / dkt047 , PMID 23429641 .
- ^ Federal Ministry of Health: MRSA , information from the Ministry of May 29, 2019; accessed December 1, 2019.
- ↑ Robert S. Daum: Removing the Golden Coat of Staphylococcus aureus . In: New England Journal of Medicine 359 (1), July 3, 2008, pp. 85-87, doi : 10.1056 / NEJMcibr0803278 , PMID 18596277 .
- ↑ Chia-I Liu, George Y. Liu, Yongcheng Song, Fenglin Yin, Mary E. Hensler, Wen-Yih Jeng, Victor Nizet, Andrew H.-J. Wang, Eric Oldfield: A Cholesterol Biosynthesis Inhibitor Blocks Staphylococcus aureus Virulence . In: Science 319 (5868), March 7, 2008, pp. 1391-1394, doi : 10.1126 / science.1153018 , PMID 18276850 , PMC 2747771 (free full text).
- ↑ a b Thanh Sang Vo, Dai Hung Ngo: The Health Beneficial Properties of Rhodomyrtus tomentosa as Potential Functional Food . In: Biomolecules 9 (2), 76, February 21, 2019, doi : 10.3390 / biom9020076 , PMID 30795643 , PMC 6406238 (free full text).
- ↑ Sukanlaya Leejae, Laila Hasap, Supayang Piyawan Voravuthikunchai: Inhibition of staphyloxanthin biosynthesis in Staphylococcus aureus by rhodomyrtone, a novel antibiotic candidate . In: Journal of Medical Microbiology 62 (3), March 2013, pp. 421-428, doi : 10.1099 / jmm.0.047316-0 , PMID 23242641 .
- ↑ Wipawadee Sianglum, Potjanee Srimanote, Wijit Wonglumsom, Kanokwan Kittiniyom, Supayang P. Voravuthikunchai: Proteome Analyzes of Cellular Proteins in Methicillin-Resistant Staphylococcus aureus Treated with Rhodomyrtone, a Novel Antibiotic Candidate . In: PLoS One 6 (2), February 4, 2011, doi : 10.1371 / journal.pone.0016628 , PMID 21326597 , PMC 3033880 (free full text).
- ↑ Wipawadee Sianglum, Dennapa Saeloh, Pongsri Tongtawe, Natthakul Wootipoom, Nitaya Indrawattana, Supayang Piyawan Voravuthikunchai: Early Effects of Rhodomyrtone on Membrane Integrity in Methicillin-Resistant Staphylococcus aureus . In: Microbial Drug Resistance 24 (7), September 1, 2018, pp. 882-889, doi : 10.1089 / mdr.2016.0294 , PMID 29215320 .

