Verbenols
The verbenols (stress on the third syllable: verbs o le , also 2-pinene-4-ole ) form a group of substances of bicyclic monoterpenes - alcohols .
Representative
Various isomeric forms of verbenols are known. In the cis isomer , the two methyl groups (-CH 3 ) are on the same side of the carbon ring as the hydroxyl group (-OH), in the trans isomer on opposite sides. In turn , there are enantiomers of each form that have optical activity , i.e. when light passes through, they rotate the position of the plane of linearly polarized light . All verbenols are monohydric, secondary alcohols, since the carbon atom that carries the hydroxyl group is bonded to two other carbon atoms.
| Verbenols | ||||||||
| Surname | (+) - trans -Vbenol | (-) - trans -Vbenol | (+) - cis -Vbenol | (-) - cis -Vbenol | ||||
| Structural formula |

|

|

|
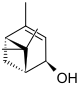
|
||||
| other names | (1 R , 2 S , 5 R ) -4,7,7-trimethyl bicyclo [3.1.1] hept-3-en-2-ol |
(1 S , 2 R , 5 S ) -4,7,7-trimethyl bicyclo [3.1.1] hept-3-en-2-ol |
(1 S , 2 S , 5 S ) -4,7,7-trimethyl bicyclo [3.1.1] hept-3-en-2-ol |
(1 R , 2 R , 5 R ) -4,7,7-trimethyl bicyclo [3.1.1] hept-3-en-2-ol |
||||
| CAS number | 22339-08-8 | 19890-02-9 | 18881-04-4 | 13040-03-4 | ||||
| PubChem | 89664 | 88298 | 87839 | 164888 | ||||
| Molecular formula | C 10 H 16 O | |||||||
| Molar mass | 152.24 g mol −1 | |||||||
| Physical state | liquid | |||||||
| Brief description | clear, colorless liquids with an oily consistency and a peculiar smell | |||||||
| boiling point | 88 ° C at 1.199 kilopascals | |||||||
|
GHS labeling |
|
|
|
|
||||
| H and P phrases | see above | see above | 315-319-335 | see above | ||||
| see above | see above | no EUH phrases | see above | |||||
| see above | see above | 261-305 + 351 + 338 | see above | |||||
Occurrence, properties and use
Verbenols are found in turpentine and incense . Together with myrcene , verbenone and ipsdienol, they are aggregation pheromones of the bark beetle .
Because of the high price, verbenols are not used as pure substances in perfumery, but only as a mixture in the form of essential oils . Because of the pheromone properties, they are used in insect traps, for example . Verbenole can be part of pesticides to be. There is no approval for this in the EU countries, but plant protection products with ( S ) - cis -beneol as an active ingredient are available in Switzerland .
literature
- Jean Pierre Vité, Wittko Francke: Forest protection against bark beetles: From the catch tree to the trap , in: Chemistry in our time , 19 (1), 11-21, (1985); doi : 10.1002 / ciuz.19850190103 .
- Chemical Abstracts (37, 361 (1943); 57, 16772 (1962)): Verbenol representation
Individual evidence
- ↑ Data sheet (S) -cis-Verbenol from Sigma-Aldrich , accessed on May 11, 2011 ( PDF ).
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on 4,6,6-trimethyl-bicyclo [3.1.1] hept-3-en-ol, ((S) -cis-verbenol) in the EU pesticide database ; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on December 7, 2019.
