Myrcene
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
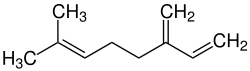
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Myrcene | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 10 H 16 | |||||||||||||||
| Brief description |
colorless to yellowish liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 136.24 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.79 g cm −3 |
|||||||||||||||
| Melting point |
<−80 ° C |
|||||||||||||||
| boiling point |
167 ° C |
|||||||||||||||
| Vapor pressure |
2.78 h Pa (at 25 ° C) |
|||||||||||||||
| solubility |
almost insoluble in water (1 mg l −1 at 20 ° C) |
|||||||||||||||
| Refractive index |
1.4697 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Myrcene (stress on the second syllable: Myrc e n , with the systematic name 7-methyl-3-methylene-1,6-octadiene ) is a tri-unsaturated acyclic monoterpene hydrocarbon and a component of many essential oils .
Occurrence

Myrcene is common in plants, including pine ( Pinus species), juniper , ginger plants ( Amomum species), mint ( Mentha species), sage , caraway , mango , fennel , tarragon , dill , mugwort ( Artemisia vulgaris ), angelica ( Angelica archangelica ), hops and hemp and many others.
Myrcene is also a pheromone of the bark beetle (Scolytidae), which attracts insects like verbenol . The antagonist that scares off beetles is verbenone .
Properties and manufacture
Myrcene is a colorless to slightly yellow liquid that boils at 167 ° C. The flash point is 39 ° C. Myrcene is readily soluble in solvents such as ethanol , chloroform and ether , but not in water. Myrcene is made from turpentine oil by pyrolysis of β- pinene .
use
Myrcene is used in the production of smells and flavors that are used in perfumery and pharmacy. Other acyclic monoterpenes such as halomons are also synthesized from myrcene.
toxicology
The IARC classified myrcene as a possible carcinogen in 2017.
Individual evidence
- ↑ Myrcene data sheet (PDF) from Merck , accessed on March 4, 2010.
- ↑ a b c d e f g h Entry on 7-methyl-3-methylenocta-1,6-diene in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ^ RT O'Connor, LA Goldblatt: Correlation of Ultraviolet and Infrared Spectra of Terpene Hydrocarbons , in: Anal. Chem. , 26 , 1954, pp. 1726-1737; doi : 10.1021 / ac60095a014 .
- ↑ Yann Grosse, Dana Loomis, Kathryn Z Guyton, Fatiha El Ghissassi, Véronique Bouvard, Lamia Benbrahim-Tallaa, Heidi Mattock, Kurt Straif: Some chemicals that cause tumors of the urinary tract in rodents. In: The Lancet Oncology . 18, 2017, pp. 1003-1004, doi : 10.1016 / S1470-2045 (17) 30505-3 .


