Halomon
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
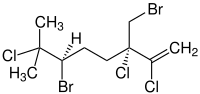
|
|||||||||||||
| General | |||||||||||||
| Surname | (-) - Halomon | ||||||||||||
| other names |
(3 S , 6 R ) -6-Bromo-3- (bromomethyl) -2,3,7-trichloro-7-methyl-1-octene ( IUPAC ) |
||||||||||||
| Molecular formula | C 10 H 15 Br 2 Cl 3 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 401.39 g · mol -1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
49-50 ° C |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Halomon is a chemical compound that belongs to the group of halogenated monoterpenes and has a pronounced and selective toxic effect on tumor cells. Since it occurs in red algae , it is one of the secondary plant substances .
History, occurrence, manufacture
Halomon was discovered in 1992 together with other halogenated monoterpenes in the red alga Portieria hornemannii . The extraction of halomon from the red alga proves to be very costly and not very promising, since not all strains of this alga produce halomons, and there are also strong fluctuations in halomons production within one strain. To extract around 400 mg of halomon, around 2 to 4 kg of this filigree red alga are required. In addition to the halomone, other structurally related compounds are also obtained during the extraction, some of which also have a strong toxic effect on tumors.
In the years 1997 to 2000, several synthetic routes to the halomon structure were described.
pharmacology
Halomon is one of the few substances that is toxic to all 60 tumor cell lines at the National Cancer Institute . While leukemia and melanoma cell lines are less sensitive, brain, kidney and colon cancer cells show a response rate that is up to a hundred times higher.
credentials
- ↑ Entry on Halomon. In: Römpp Online . Georg Thieme Verlag, accessed on December 29, 2014.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b Richard W. Fuller, John H. Cardellina, Yoko Kato, Linda S. Brinen, Jon Clardy, Kenneth M. Snader, Michael R. Boyd: A pentahalogenated monoterpene from the red alga Portieria hornemannii produces a novel cytotoxicity profile against a diverse panel of human tumor cell lines. In: Journal of Medicinal Chemistry. 35, 1992, pp. 3007-3011, doi : 10.1021 / jm00094a012 .
- ↑ Schlama, Thierry; Baati, Rachhid; Governor, Veronique; Valleix, Alain; Flack, John R .; Mioskowski, Charles: Total synthesis of (±) -halomon by a Johnson-Claisen rearrangement. Angewandte Chemie, International Edition 15/37/1998. S. 2085-2087 doi : 10.1002 / (SICI) 1521-3773 (19980817) 37:15 <2085 :: AID-ANIE2085> 3.0.CO; 2-J .
- ↑ Jung, Michael E .; Parker, Michael H .: Synthesis of Several Naturally Occurring Polyhalogenated Monoterpenes of the Halomon Class. Journal of Organic Chemistry 21/62/1997. Pp. 7094-7095 doi : 10.1021 / jo971371 + .
- ↑ Sotokawa, Takayuki; Noda, Takeshi; Pi, Sun; Hirama, Masahiro: A three-step synthesis of halomon. Angewandte Chemie 19/39/2000. Pp. 3430-3432 doi : 10.1002 / 1521-3773 (20001002) 39: 19% 3C3430 :: AID-ANIE3430% 3E3.0.CO; 2-3 .