Pyrethrins

Pyrethrins are a group of natural substances that are responsible for the insecticidal effects of pyrethrum . They are made from certain types of chrysanthemums . Pyrethrins are isolated from pyrethrum extract and used as insecticides for crop protection, pest control and in medicine.
history
In 1917 the US Navy developed a process for the production of a pyrethrum extract, in which the ground chrysanthemum flowers, which were previously used directly as "insect powder", were extracted with petroleum . In formulations based on this extract, the active ingredients could now be used by spraying to combat flies and mosquitoes.
The basic structure of pyrethrins as esters of two different organic acids ("chrysanthemum acid" and "pyrethric acid") with, as initially assumed, a ketone ("pyrethrolone") was elucidated between 1910 and 1916 by Hermann Staudinger and Leopold Ružička , but only published in 1924 . In 1944 it was recognized that the substances previously known as pyrethrin I and pyrethrin II were mixtures of substances. In addition to the pyrethrolone, cinerolone could be identified as the second pyrethrin-forming ketone, with which the known pyrethrins were expanded to include cinerin I and cinerin II. With the identification of jasmolone as the third ketone in 1966, Jasmolin I and Jasmolin II were also known. The six pyrethrins became the model for the synthetically produced pyrethroids .
composition
| Pyrethrins | ||||
| Surname | Pyrethrin I. | Pyrethrin II | ||
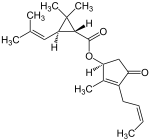
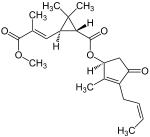
| Structural formula |  |
|
||
| CAS number | 8003-34-7 (mixture) | |||
| ? | ? | |||
| PubChem | 6433154 | 6433155 | ||
| Brief description | yellow to dark brown viscous liquid | |||
| Molecular formula | C 21 H 28 O 3 | C 22 H 28 O 5 | ||
| Molar mass | 328.45 g mol −1 | 372.45 g mol −1 | ||
| Physical state | liquid or solid | |||
| boiling point | 100–200 ° C (10 Pa ) | |||
| solubility | low in water | |||
|
GHS labeling |
|
|||
| H and P phrases | 332-312-302-410 | |||
| no EUH phrases | ||||
| 273-280-501 | ||||
| MAK value | not fixed | |||
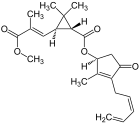
The two most important pyrethrins are pyrethrin I and pyrethrin II . These are the esters of (+) - trans-chrysanthemum acid and (+) - trans-pyrethric acid with the hydroxyketone (+) - pyrethrolone . In addition, pyrethrum extracts also contain the cinerins Cinerin I and Cinerin II, as well as the jasmolines Jasmolin I and Jasmolin II.
| Proportion | Structural formula | |
|---|---|---|
| Pyrethrin I. | 35% | |
| Pyrethrin II | 33% | |
| Jasmolin I | 5% | |
| Jasmolin II | 4% | |
| Cinerin I. | 10% | |
| Cinerin II | 14% |
Pyrethrin I is the single compound with the highest insecticidal effectiveness. Pyrethrin II has a weaker effect, but it occurs particularly quickly. Pyrethrum flowers from Kenya contain an average of 1.3% pyrethrins, which is around 2-3 mg per flower, sometimes 4 mg. The flowers are dried and pulverized after harvest. The pyrethrins are extracted therefrom with solvent mixtures such as methanol / kerosene , petroleum ether / acetonitrile or petroleum ether / nitromethane . With petroleum ether / nitromethane, a 90 percent mixture of the six pyrethrins is obtained.
Little is known about the biosynthesis of pyrethrins. Mevalonic acid is possibly a precursor to the formation of chrysanthemum acid .
effect
Pyrethrins are contact insecticides , for example, against aphids , whiteflies , spider mites , wool and mealybugs , leafhoppers and beetles - larvae . They work against eggs, larvae and adult stages. Pyrethrins are not beneficial to beneficial organisms. Their effect on insects occurs within a few minutes, one speaks of a "knock-down" effect. The dose required for the "knock-down" is low, but many of the insects affected manage to break down the pyrethrins and recover. To prevent this detoxification, pyrethrins are mixed with the synergist piperonyl butoxide .
Natural occurrence
In insect repellent herb pyrethrins are included, of course.
use
Plant protection
Pyrethrins are the effective ingredient in many home garden and greenhouse insect sprays. They are also available in the form of cold misting agents, powders and as emulsion or suspension concentrates . Sprays and concentrates often contain rapeseed or sesame oil as a carrier substance. Piperonyl butoxide is usually added as a synergist . Because of their rapid action, pyrethrins are an additive in some pesticides containing dichlorvos .
Pyrethrins are approved as an active ingredient in plant protection products in many EU countries, including Germany, Austria and Switzerland. In Austria and Switzerland, pyrethrins are also approved for the protection of stored products , for example to control grain weevils in grain stores.
Pest Control
Pyrethrins are used as insecticidal agents against a number of insect pests and as repellants .
According to EU Directive 98/8 / EC of February 16, 1998, biocidal products should only be approved if their active ingredients have been included in the appendix (Appendix I, IA and IB) of the aforementioned directive (for the defined product type). According to the transitional regulation (Art. 16 (1) of Directive 98/8 / EC), however, the placing on the market of biocidal products that do not contain the active substances listed in the annex to Directive 98/8 / EC was permitted, provided that these active substances were released on May 14th 2000 were already on the market (also called "old active ingredients"). According to EU regulation 1896/2000 of September 7, 2000, manufacturers who wanted to apply for the inclusion of an "old active substance" in Annexes I, IA and IB had to report the relevant active substance for notification for the corresponding product type by March 28, 2002 to have. This period was extended from September 25, 2002 to January 31, 2003 by EU regulation 1687/2002.
The active ingredient pyrethrins was subsequently reported for product type 18 (insecticides) and 19 (repellants) and added to the list of notified active ingredients.
Until the final decision on inclusion or non-inclusion in Annex I, IA and IB of EU Directive 98/8 / EC (as of March 26, 2013, the decision on this is still pending), products with the active ingredient pyrethrins for pest control may remain on the market.
toxicology
Pyrethrins have a neurotoxic effect on sensory as well as motor nerves. The lethal dose for pyrethrin I and II for rodents is in the range from 130 to over 600 mg / kg body weight . If the substances are administered in several small doses over 12 to 48 hours, the lethal dose is up to 2900 mg / kg body weight. A two year old child died after eating 14 grams of pyrethrum powder. There was also fatal poisoning from inhaled pyrethrin aerosol .
When pyrethrins are applied to the skin, this often results in a brief feeling of cold due to local paresthesia . The absorption of the substances through the skin surface is low. Pyrethrins irritate the eyes and the mucous membranes, but are only slightly irritating to the skin . Unpurified pyrethrum extract is skin sensitizing , but this is not due to the pyrethrins it contains.
Individual evidence
- ↑ Albert Gossauer: Structure and reactivity of biomolecules. Verlag Helvetica Chimica Acta, Zurich 2006, ISBN 978-3-906390-29-1 , p. 132.
- ^ Antonia Glynne-Jones: Pyrethrum. In: Pesticide Outlook. October 2001, pp. 195–198, (PDF; 274 kB, English), online at researchinformation.co.uk, accessed January 5, 2017.
- ^ H. Staudinger, L. Ruzicka: Insecticidal substances I – X. In: Helvetica Chimica Acta 7. No. 1, 1924, pp. 177-458.
- ^ FB La Forge, WF Barthel: Constituents of Pyrethrum Flowers. XVI: Heterogenous Nature of Pyrethrolone. In: The Journal of Organic Chemistry. 9, No. 3, 1944, pp. 242-249.
- ↑ PJ Godin et al .: The jasmolins, new insecticidally active constituents of Chrysanthemum cinerariaefolium Vis . In: Journal of the Chemical Society C: Organic. 1966, pp. 332-334.
- ↑ a b Entry on Pyrethrum. In: Römpp Online . Georg Thieme Verlag, accessed on January 5, 2017.
- ↑ a b c d e Entry on pyrethrins in the GESTIS substance database of the IFA , accessed on January 5, 2017(JavaScript required) .
- ↑ Data sheet Pyrethrum extract from Sigma-Aldrich , accessed on January 5, 2017 ( PDF ).
- ^ A b Klaus Naumann: Synthetic Pyrethroid Insecticides: Structures and Properties. 1990.
- ^ Directorate-General for Health and Food Safety of the European Commission: Entry on pyrethrins in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; Retrieved January 5, 2017.
- ↑ EU: Directive 98/8 / EG of February 16, 1998 on the placing of biocidal products on the market . In: Official Journal of the European Communities L 123/1 of April 24, 1998, accessed on January 5, 2017.
- ↑ EU: Regulation (EC) No. 1896/2000 on the first phase of the program in accordance with Article 16, Paragraph 2 of Directive 98/8 / EC on biocidal products . In: Official Journal of the European Communities L 228/6 of September 8, 2000, accessed on January 5, 2017.
- ↑ EU: Regulation (EC) No. 1687/2002 on an additional period for the notification of certain active substances . In: Official Journal of the European Communities L 258/15 of September 26, 2002, accessed on January 5, 2017.
- ↑ EU: Regulation (EC) No. 2032/2003 on the second phase of the ten-year work program on placing biocidal products on the market . In: Official Journal of the European Communities L 307/1 of November 24, 2003, accessed on January 5, 2017.
- ↑ Persian insect flower (Chrysanthemum coccineum). Retrieved January 2, 2019.