Natural rubber

Natural rubber , formerly simply called rubber , also known as rubber elasticum or Resina elastica , is a rubber-like substance in the milky sap (latex) of many different rubber plants. The latex containing rubber is mostly in the form of a milky liquid, but the plants can also contain it in semi-solid form. The milk juice tastes pleasant, similar to sweet cream and is enjoyable.
etymology
The German word rubber is a loan word from the French caoutchouc and goes back to an indigenous language of Peru via the Spanish cauchuc , formerly caucho . In Tupi or Quechua , the term caa ochu , formed from the words caa 'tree', 'wood' and ochu 'tear', 'blood', stands for “weeping”, “bleeding wood” or “tears of the tree”. With regard to the milky sap that flows when the bark is injured , the term was adopted in Spanish as caucho , also denoting a rubber collector. The French, 1743-1745 the Amazon prepared end of La Condamine called the collected product caoutchouc ; He called the milky juice latex , in Spanish leche 'milk', or in Latin latex 'liquid' (from ancient Greek látax 'drops').
Originally rubber was the name for natural rubber; With the development of synthetic rubbers, all highly polymeric substances that are amorphous at room temperature , have a low glass transition temperature and have increasing plasticity when the temperature rises are called “rubber” today . Today natural rubber makes up less than half of the rubber produced; 60% of global rubber demand is covered by synthetic rubber.
origin
The natural rubber is today, both in Southeast Asia as in Central and South America mainly from the original only in the Amazon basin occurring rubber tree ( Hevea brasiliensis won). Over 99% of natural rubber today comes from this type as Hevea rubber , which is also called para-rubber . The prefix Para- stands for the Brazilian state of Pará , from which Hevea rubber previously came.
properties
The latex of the rubber tree is a colloidal dispersion of about one third of rubber in an aqueous solution (serum). The main component of the colloid is a polymer made of isoprene units, the cis -1,4- polyisoprene ; other substances are proteins and resins that stabilize the colloids. The specific gravity of the colloid is around 0.93-0.96, that of the serum is 1.02.
The Hevea rubber obtained from the latex contains about 2.8% protein, 2.8% resin, 0.2 to 0.6% water and about 0.38% mineral components. The cleaned rubber is brownish and somewhat translucent. It becomes brittle on cooling to 3 ° C or below. When heated to 145 ° C it becomes sticky, above 170 ° C it melts. It is sensitive to oxidizing agents and aggressive media. Rubber is soluble in gasoline, chlorinated hydrocarbons and oils. In hot water it can neither be greatly softened nor made kneadable.
Substances that consist of plants with predominantly trans- or mixed- configured 1,4-polyisoprene, such as balata , gutta-percha or chicle , are not designated as natural rubber.
Other sources
Many other plant species provide cis -1,4-polyisoprene of varying quality, but are little or not used. Some of the thousands of species in around 20 plant families are:
Hevea benthamiana , Hevea guianensus (Para-rubber), Manihot carthaginensis subsp. glaziovii (Ceara rubber), Manihot dichotoma (Jeque rubber), Castilla elastica (Panama rubber), Ficus elastica (Indian rubber), Funtimia elastica (Lagos rubber), Landolphia kirkii (Landolphia rubber), Landolphia gentphiailli , Landolphiailli heudelotii , Landolphia owariensis (Landolphia- or Madagascar rubber), Crytostegia grandiflora , Crytostegia madagascariansis (Madagascar rubber), Parthenium arge Tatum ( guayule ), Taraxacum kok-saghyz (dandelion rubber), prickly lettuce ( Lactuca serriola ) Willoughbya spp . (Borneo rubber), Hancornia spp. (Bahia or Mangabeira rubber) and many more.
history
The chemical molecular formula of natural rubber was found by Michael Faraday in 1826 and the isoprene component by Charles Hanson Greville Williams in 1860. The correct conception of natural rubber as a macromolecule comes from Hermann Staudinger (1920), although he had a forerunner in Samuel Pickles (1906).
Beginnings in Central America
The oldest known objects made of rubber date from around 1600 BC. The peoples of Central American Mesoamerica and the indigenous peoples of the Amazon region already used natural rubber in many ways in pre-Columbian times. The best known is the Mesoamerican ball game with a solid rubber ball. Because of its water-repellent properties, fabric was also coated with rubber. The Maya are, for example, their feet are provided with a limited durable rubber coating. In addition, the rubber was useful in everyday life. Hoses, vessels, torches and even items of clothing were made with it. It was also used as an offering at religious gatherings, on days of sacrifice and on solemn occasions.
Start of use in Europe
After the discovery of America by the Europeans, rubber was initially only known to a few people in Europe, such as Emperor Charles V , to whom an Aztec ball game team was demonstrated by Hernán Cortés . It was only through books that knowledge became increasingly public. In 1615 Juan de Torquemada described in "De la Monarquia Indiana" how the Indians made objects water-repellent, the Conquistador Bernal Díaz del Castillo described Aztec ball players around 1520. However, this report was only discovered and published in a library in Madrid in 1632.
Charles Marie de La Condamine , on a scientific expedition for the Paris Academy of Science from 1735 to 1745 in the Amazon region, observed how rubber was used and described the Native American way of making it. This triggered further reports and the first European attempts with the new material : a solvent for solid rubber was found in 1761, the eraser was created around 1770, and Samuel Peal's first patented process in 1791 for applying rubber dissolved in turpentine to fabric, the first in 1824 Raincoat or Macintosh and the "Wellington boots". These early Wellington boots were made famous in early 19th century England by Arthur Wellesley, 1st Duke of Wellington . Despite this and other successes, the material was still difficult to use because it began to stick when it was very hot and became brittle when it was cold.
Rubber boom and crash
Invention of rubber

The ancient Mesoamericans did not know the process of vulcanization. But by adding tree and plant sap they achieved the conversion of the plastic rubber to an elastic one.
In 1839, Charles Goodyear invented the process of vulcanization, by means of which plastic rubber can be converted into elastic rubber. This offered many new application possibilities, so that in the years from 1839 to 1910 there was a rubber boom in the Amazon region that made the cities of Manaus and Belém the richest regions in Brazil at the time . At that time, the Teatro Amazonas in Manaus, which opened with La Gioconda by Amilcare Ponchielli on January 7, 1897 , and the 364 km long Madeira-Mamoré Railway (EFMM) were created. This was supposed to transport rubber from areas of the Amazon, which are difficult to reach by ship, to Porto Velho on the Rio Madeira . The railway connection was even mentioned in the Petrópolis Treaty between Bolivia and Brazil, as an extension of the route from the Brazilian border town of Guajará-Mirim on the Río Mamoré to the Bolivian city of Riberalta was agreed. However, this was never built because the rubber boom ended before that.
Rubber tree plantations

After rubber in the form of rubber had become an important material, attempts were made to grow rubber trees in plantations . This did not succeed in South America because the fungus Microcyclus ulei prevented this production method. However, the English were able to build plantations in their colonies in Asia ( Microcyclus ulei has not yet been able to establish itself in Asia, but other types of fungi that can be controlled with fungicides .)
As early as 1876 the Englishman Henry Wickham had smuggled around 70,000 rubber seeds from Brazil into British Ceylon (now Sri Lanka), but it was not until the beginning of the 20th century that larger quantities of rubber from Asia came onto the market. Another important production area was tropical Africa. Especially in the Congo Free State under the rule of the Belgian King Leopold II , the local population was forced to collect rubber using brutal methods (" Congo Abomination "). In the French colonial areas such as Gabon and the Central African Republic , the inhabitants were also exploited in this way.
With the additional plantations outside of Brazil, the rubber demand could be better covered, so that the price fell and the rubber boom in Amazonia came to an end. Although the great demand during the First World War once again led to an upswing, this did not last. In addition to the Brazilians, the British also suffered from the lower price, which is why they came up with the Stevenson Plan in 1922 , a rubber cartel that was primarily at the expense of the largest consumer, the USA. It was at this time that the owner of the Ford plant , Henry Ford , came up with a plan to grow rubber himself in Brazil. In today's Fordlândia in the municipality of Aveiro , Ford employed up to 5,000 workers in the twenties, but the project failed due to various difficulties, for example severe infestation by the fungus Microcyclus ulei , which occurs in Brazil . In 1934, the International Rubber Regulation Agreement was another attempt to stabilize the rubber price.
Rubber shortage during the world wars
Synthetic rubber alternatives in Germany
During the First and Second World Wars , the German Reich lost access to its rubber sources, which encouraged the search for alternatives. During the First World War Fritz Hofmann produced so-called methyl rubber ( synthetic rubber ), a rubber substitute, from dimethyl butadiene . Rubber also became scarce during the Second World War, but this time not only for the European Axis powers , but also for the Allies , since the Asian plantations had been conquered by Japan. In the German Reich, the chemical company IG Farben produced styrene-butadiene rubber under the name Buna in the Buna works in Schkopau from 1935 onwards . Lignite was used as the raw material in Schkopau , for example , while the necessary hydrogen came from the neighboring Leunawerk .
Synthetic rubber alternatives in the US
From 1940 onwards, the state-owned US American Rubber Reserve Company was storing natural rubber because the USA feared a delivery stop in the event of an attack by Japan in Asia. When this happened, the USA began to build 15 state-funded factories for Buna rubber from 1941. The patents for this styrene-butadiene rubber were held by Standard Oil of New Jersey , which, due to an agreement with IG Farben, refused to release the Buna patents for the American market. As a result, a commission of inquiry accused the company of a “continued conspiracy in favor of Germany” and Harry S. Truman spoke of “treason” at a press conference. The US Congress decided to release the Buna patents for America. In 1943, US production of 185,175 t of “government rubber” exceeded German production of 110,569 t for the first time and was increased to over 730,000 t / year by the end of the war.
Natural rubber alternatives
There have also been attempts to use other rubber-producing plants. During the Russian campaign , the German Wehrmacht captured Soviet research material on the use of Russian dandelion ( Taraxacum kok-saghyz Rodin ) for rubber production. There were German plans to grow dandelions on 1,200 km² in Eastern Europe in 1944, as the roots of this dandelion contain between 6% and 10% rubber. But this became impossible because of the course of the war. In the USA, the dwarf shrub guayule ( Parthenium argentatum ) was investigated as a substitute plant during the Second World War. Here too, rubber is mainly concentrated in the roots with a proportion of 5% to 7%. An EU research project, EU-PEARLS, which will run until 2012, began in April 2008, in which both plant species are researched. In the meantime, the Continental company is again researching the use of dandelion rubber as a raw material for car and truck tires. Guayule rubber is also used today for antiallergic rubber products and also for tires.
Extraction and trading
Extraction
Natural rubber is mainly obtained from the rubber tree. The liquid, also known as latex or milky sap, is usually released by scratching the tree bark because it is contained in liquid in tubular milk tubes and collected in containers. However, this is not always the case with some other types, which also supply rubber.
| Composition of latex (milk juice from Hevea brasiliensis ) |
|---|
| 60-75% water |
| 25-35% rubber |
| 1.5-2.5% resins |
| 1.5–2% protein |
| 0.5–1% minerals |
The majority of natural rubber is obtained in plantations.
In Brazil it is still only obtained at great expense as a collector's product ( extractivism ). This ecologically relatively harmless, sustainable management provides the locals with income. Chico Mendes , murdered in 1988, was involved in the development of the concept of the reserve . In 2007 there were 65 designated rubber collecting areas (Reservas Extrativistas, RESEX) in the Amazon with a total area of 117,720 km². This is 2.3% of the total area of the Amazon. The tapping of natural rubber, the extraction of edible oils, fishing and, on a small scale, the felling of trees are permitted there. It is not uncommon for illegal activities in the reserve to lead to conflicts.
Commercial form
In addition to natural rubber, which is traded as a bale or powder, stabilized latex concentrate is also available on the market. Stabilized latex concentrate is easier to disperse during processing and can be mixed better with other substances. The latex is prevented from coagulating on site with ammonia and treated antibacterially with other substances. The dispersion is concentrated by centrifugation, evaporation in vacuo, or creaming.
In order to obtain rubber, the latex is made to clot (coagulate). In the past this was done by stirring the heated latex, today it is done in factories by adding acids. The moist solid is then smoked and then washed, dried and shaped in various ways. A commercial variant is sheet rubber . The natural rubber is coagulated with a weak formic or acetic acid. The rubber is then rolled in a rolling mill into a smooth strip several millimeters thick and about half a meter wide, with the last roller imprinting a characteristic pattern. Traditional commercial varieties of this variant are "smoked sheets" (dried in the smoke) and "air dried sheets" (dried smoke-free). Another variant is crepe rubber . Traditional commercial varieties are "pale crepes" (selected quality). In this case, the latex is coagulated with sodium hydrogen sulfite . The rubber runs through grooved rollers, followed by a few smooth rollers. The second variant is "brown crepes" (lower quality) made from high-quality rubber waste.
After separating into about 1 meter long "sheets" or "crepes" are sorted according to quality and sold as bales of about 100 kg . Today, natural rubber produced according to nationally standardized processes is the most common. These methods are, for example, Thai Tested Rubber (TTR), Standard Indonesian Rubber (SIR) or Standard Malaysian Rubber (SMR) and the like. a.
World production
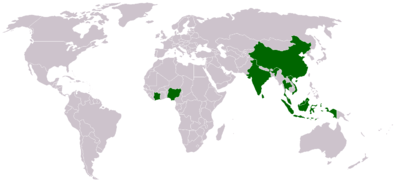
The five most important producers of natural rubber today are Thailand , Indonesia , Malaysia , India and the People's Republic of China . The largest African producers are Ivory Coast , Nigeria and Liberia . World production in 2003 amounted to 7.6 million t (dry weight), of which 80% was exported. The main customers are the United States , Japan , China , Germany and France .
| rank | country | Production (in thousand tons ) |
rank | country | Production (in thousand tons) |
|
|---|---|---|---|---|---|---|
| 1 | Thailand | 3030 | 10 | Brazil | 96 | |
| 2 | Indonesia | 1792 | 11 | Sri Lanka | 92 | |
| 3 | Malaysia | 1000 | 12 | Philippines | 88 | |
| 4th | India | 694 | 13 | Guatemala | 50 | |
| 5 | China | 550 | 14th | Cambodia | 46 | |
| 6th | Vietnam | 391 | 15th | Cameroon | 46 | |
| 7th | Ivory Coast | 123 | 16 | Myanmar | 36 | |
| 8th | Nigeria | 112 | 17th | Mexico | 23 | |
| 9 | Liberia | 108 |
Source: Handelsblatt - The world in numbers (2005)
Vietnam has expanded the cultivation of natural rubber significantly in recent years. In 2010, exports rose to a value of 782,200 tons with a world market value of US $ 2.38 billion. The area under cultivation for natural rubber was 740,000 hectares in 2010 and thus ranked fifth after Thailand, Indonesia, Malaysia and India.
Chemical structure
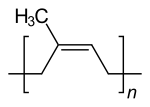
Natural rubber is a polymer made from the monomer isoprene (2-methyl-1,3-butadiene) and has an almost uniform structure with a cis- 1,4 linkage. It is assigned to the polyterpenes . The mean molar mass of natural rubber is extremely high at around 500,000 to 2 million g · mol −1 .
Material properties
Physical Properties
Unvulcanized natural rubber is visco-elastic , it deforms permanently under a longer applied force and does not completely return to its original shape after the end of the force. The reason for this is that the polymers are not covalently linked to one another.
After vulcanization, natural rubber (or now rubber) shows two important areas of elasticity due to the crosslinking of the polymer chains. At low temperatures it is now elastic - so it completely returns to its original shape after a force has been applied. At high temperatures, on the other hand, the material is still viscoelastic.
Below their glass transition temperature , both rubber and rubber become hard and brittle.
Natural rubber is much more durable than the common synthetic variants and is therefore used, for example, for heavily stressed tire applications in the construction industry. This advantage is due to shear-induced crystallization - a spontaneous, reversible stiffening of the material under mechanical stress (running over sharp stones, broken glass, etc.). Synthetic and natural rubber are mixed in conventional car tires .
Allergenic properties
An allergy to para rubber can occur, the so-called latex allergy . The actual allergen is a trace of a protein it contains . Although this allergy is relatively widespread, the exact prevalence (frequency of the disease) can only be estimated at between 3 and 20%. It is known, however, that people who often come into contact with natural rubber represent a risk group that is significantly more likely to suffer from this allergy. These are, for example, doctors, operating room nurses, nurses, but also children who have to undergo frequent operations. An alternative is to use synthetic rubber or guayule rubber . However, some plastics contain natural latex without labeling, which can be particularly problematic for patients with this allergy.
Further processing

processing
Natural rubber (like synthetic rubber) can be vulcanized . This means that it is crosslinked with sulfur or with peroxides , metal oxides and others ( EPM , EVA , neoprene), but it can also only be crosslinked thermally ( styrene-butadiene rubber (SBR) and nitrile rubber (NBR)). The sulfur content also determines the hardness; 4–5% soft, 25–30% hard.
Depending on the use, the rubber is supplemented by additives such as:
- Masticating aid (mastication or mastication is the mechanical tearing of longer rubber polymer chains with the help of rollers or kneading machines); chemical agents help break up the chain molecules
- Carbon blacks with a large internal surface, e.g. B. to increase the UV resistance and abrasion resistance of car tires,
- Plasticizers ,
- Factice (cross-linked mineral or vegetable oils with rubber-like properties),
- Crosslinking chemicals,
- Anti-aging agents,
- Flame retardants ,
- Pigments or dyes .
Natural rubber can be used as the sole polymer or in mixtures with synthetic rubbers. The disadvantage is that natural rubber embrittles or can dissolve on contact with sunlight , UV light or fats .
Products
Natural rubber can be processed into a wide variety of products:
- 70% of the rubber is used for the production of car tires , 12% of the rubber for the production of latex products, 8% for the production of technical products.
- Binders for paper coating , carpet backing and dipped articles such as thin gloves.
- In its foamed form, rubber is used for mattresses .
- Rubber can be further processed into pore rubber (also known as foam rubber ), in which the elastic foam can have pore structures ranging from completely closed to completely open . In this way not only material and thus costs can be saved, but also certain mechanical properties of the profile, such as hardness , can be changed.
- Another important application is sealing profiles made of rubber. Due to its favorable weathering properties, ethylene-propylene-diene rubber (EPDM) is mainly used for this . The sealing systems are produced by extrusion and often flocked , laminated and / or painted in a subsequent finishing process .
- Garments and other articles made of rubber ( latex clothing ) or rubber irritate the skin and other senses (smell, appearance) in a special way (warmth - cold, moisture, emphasis on body shapes), and thus serve rubber fetishism .
- Other uses are the production of thin films for condoms , gloves , or balloons ( Dipped Goods ) and in thicker films for the production of casting molds , truck and passenger car tires , motor bearings , as well as various rubber / metal compounds.
- In combination with sulfur, natural rubber is vulcanized into ebonite , which u. a. is used in the manufacture of writing implements and mouthpieces ( wind instruments , tobacco pipes ).
- With various additions it becomes gum processed
- It is used as an eraser or type rubber in drawing and writing techniques.
See also
literature
- Georg Abts: Introduction to rubber technology. Hanser, Munich / Vienna 2007, ISBN 978-3-446-40940-8 .
- Fritz Röthemeyer, Franz Sommer, Peter Bartholomei a. a .: rubber technology. Materials - Processing - Products. 2nd, revised edition. Hanser, Munich / Vienna 2006, ISBN 3-446-40480-5 .
- Werner Hofmann, Heinz Gupta, M. Burger u. a .: Handbook of Rubber Technology. Gupta, Ratingen 2001, ISBN 3-9803593-2-8 .
- Christian Mähr : From alcohol to sugar - twelve substances that changed the world. DuMont, Cologne 2010, ISBN 978-3-8321-9549-6 .
Fiction representations
- Ilja Ehrenburg : The Life of Cars. German edition East Berlin 1976. In the chapter Tires , dated 1929, Ehrenburg describes the battles for cheap rubber of the time (pp. 229-251).
- José Maria Ferreira de Castro : A Selva. Roman, 1930, German under the title Die Kautschukzapfer Hamburg 1933 and other editions. De Castro processes his own (devastating) experiences as a tapper, which he made in Brazil around 1910.
- Madelon Lulofs : Rubber. A novel from Sumatra. Original title: Rubber. 1931, translated by Walter Hjalmar Kotas. 1st German-language edition. Holle & Co., Berlin / C. Fr. Fleischer, Leipzig 1934. A critical, partly autobiographical description of everyday life in the rubber plantations in the Dutch colony at the time. The debut novel was noticed, translated and filmed worldwide - except in the Netherlands. Recent editions: Goldmann Taschenbuch 354, Munich 1955, bound: Schwingen Verlag, Rosenheim 1963.
Web links
- Meyer's Large Conversation Lexicon. Volume 10, Leipzig 1907, pp. 786-791: Rubber at Zeno.org .
- Pictures of the raw material rubber. on swisseduc.ch.
- A brief history of rubber. on bouncing-balls.com.
- Specialist information from German transport insurers on natural rubber on the Transport Information Service (TIS).
- Technical information from German transport insurers on synthetic rubber on the Transport Information Service (TIS).
- Internet presence of the International Rubber Study Group (IRSG). Establishment of the global rubber industry for the provision of statistical data in the field of rubber, only partially freely accessible.
- Hans-Dieter Feger: History and economic development of rubber. Summary of a diploma thesis including various illustrations, Innsbruck, March 1973, accessed on Jan. 28, 2012
Individual evidence
- ↑ Steinbüchel, Oppermann-Sanio, Ewering, Pötter: Microbiological internship. 2nd Edition. Springer, 2013, ISBN 978-3-642-25150-4 , p. 180.
- ↑ rubber. In: Lexicon of Chemistry. Spectrum of Science Publishing Company, accessed on January 22, 2018 .
- ↑ Kluge: Etymological dictionary of the German language . 23rd edition, Berlin 1995, p. 434.
- ↑ Georg Friederici : Americanist Dictionary. De Gruyter, 1947, p. 640.
- ↑ MJ Loadman: Analysis of Rubber and Rubber-like polymer. Fourth Edition, Springer, 1998, ISBN 94-010-5905-5 , p. 7.
- ↑ Shinzo Kohjiya, Yuko Ikeda: Chemistry, Manufacture and Applications of Natural Rubber. Woodhead, 2014, ISBN 978-0-85709-683-8 , pp. 30–34, limited preview in Google Book Search.
- ↑ P. Venkatachalam, N. Geetha et al. a .: Natural rubber producing plants: An overview. In: African Journal of Biotechnology. Vol. 12 (12), 2013, pp. 1297-1310, doi: 10.5897 / AJBX12.016 , online (PDF; 430 kB).
- ↑ W. Blaschek, R. Hansel u. a .: Hager's Handbook of Pharmaceutical Practice. Volume 2: Drugs A – K. 5th edition. Springer, 1998, ISBN 3-642-63794-9 , p. 839.
- ^ PH List, L. Hörhammer: Hager's handbook of pharmaceutical practice. 5. Volume, Chemicals and Drugs (H – M) , Springer, 1976, ISBN 3-642-65644-7 , p. 66 ff, limited preview in the Google book search.
- ↑ a b c d e Hans-Dieter Feger: History and economic development of rubber. ( Memento of March 18, 2014 in the Internet Archive ) Summary of a diploma thesis including various images, Innsbruck, March 1973, accessed on January 28, 2012.
- ^ D. Hosler: Prehistoric Polymers: Rubber Processing in Ancient Mesoamerica. In: Science. 284, pp. 1988-1991, doi: 10.1126 / science.284.5422.1988 .
- ↑ Microcyclus ulei (South American leaf blight of rubber). In: The Invasive Species Compendium. CABI, accessed March 8, 2017 .
- ↑ Diseases of Rubber an overview (PDF) on fao.org.
- ↑ Ford's Obsession to Rubber. An Empirical Study of Irrational Decision (PDF; 112 kB).
- ↑ Jochen Streb: The development of the synthetic rubber industry in Germany and the USA before and during the Second World War ( Memento from January 10, 2012 in the Internet Archive ) ( MS Word ; 165 kB).
- ↑ Dandelion cultivation for the production of rubber on onlinereports.ch.
- ↑ Susanne Donner: From war research to new biotechnology In: Frankfurter Allgemeine. May 4, 2008, p. 67.
- ↑ Dandelion crop database at Marburg University.
- ↑ Guayule useful plants database at Marburg University.
- ↑ EU-PEARLS: EU-based Production and Exploitation of Alternative Rubber and Latex Sources .
- ↑ Gum from the dandelion ( page no longer available , search in web archives ).
- ^ Dandelion rubber research in Anklam. September 1, 2016, accessed September 6, 2016 .
- ↑ a b c d e Transport Information Service (TIS): Natural rubber , specialist information from German transport insurers, accessed on February 25, 2010.
- ↑ Gunther Franke: Useful plants of the tropics and subtropics. Volume 1: General Plant Production , Ulmer 1995, ISBN 3-8001-2687-7 .
- ↑ Dieter Gawora, Maria Helena de Souza Ide, Romulo Soares Barbosa (ed.), Mirja Annawald (transl.): Traditional peoples and communities in Brazil. Latin America Documentation Center. Kassel University Press, Kassel 2011.
- ↑ a b Trần Thị Thuý Hoa, General Secretary of the Việt Nam Rubber Association, quoted in Việt Nam News, edition May 13, 2011, p. 15.
- ↑ Georg Abts: Introduction to rubber technology. ISBN 3446409408 p. 170 ( limited preview in Google Book Search).
- ↑ Kerstin Hoppenhaus: The Rubber Apocalypse In: Time Online .