Ethylene propylene copolymer
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
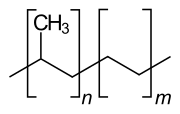
|
|||||||
| General | |||||||
| Surname | Ethylene propylene copolymer | ||||||
| other names |
|
||||||
| CAS number | 9010-79-1 | ||||||
| Monomers / partial structures | |||||||
| Type of polymer |
Thermoplastic (copolymer) |
||||||
| Brief description |
odorless white solid |
||||||
| properties | |||||||
| Physical state |
firmly |
||||||
| density |
0.9 g ml −1 at 25 ° C |
||||||
| solubility |
almost insoluble in water |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Ethylene-propylene copolymer ( abbreviation E / P , formerly also EPM , also EPR from English ethylene-propylene rubber ) is a copolymer made from ethene and propene . Areas of application are, for example, hot melt adhesives and sealants .
Properties and manufacture
The copolymerization of ethylene increases the toughness of the polymer compared to pure polypropylene. Production can take place in a homogeneous phase using Ziegler-Natta catalysts or metallocene catalysts. The amount of ethylene used ranges from 40 to 80%.
The plastic can be processed with many additives and is relatively resistant to oxidizing agents. It is used as cable insulation. Among other things, its resistance to ozone (which occurs during partial discharges ) is important here.
Furthermore, functionalized olefins such as maleic anhydride or diolefins can be polymerized in to increase the functionality of the plastic. The diolefins can optionally allow crosslinking of the polymer.
Individual evidence
- ↑ a b Safety data sheet Formolene® Polypropylene Copolymer , accessed on July 9, 2019.
- ↑ a b c data sheet poly (ethylene-co-propylene) from Sigma-Aldrich , accessed on June 15, 2011 ( PDF ).
- ↑ Compendium of Polymer Terminology and Nomenclature , IUPAC Recommendations, RSC Publishing, Cambridge, 2008, pp. 403f.
- ↑ Morphology and toughness using the example of impact-modified polypropylene materials (PDF; 146 kB) sundoc.bibliothek.uni-halle.de. Retrieved December 6, 2009.
- ↑ Bernd Tieke, Makromolekulare Chemie , 3rd edition, Wiley-VCH, Weinheim, 2014, p. 173.