Gutta-percha
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
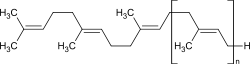 n≈100 |
|||||||
| General | |||||||
| Surname | Gutta-percha | ||||||
| CAS number | 9000-32-2 | ||||||
| Monomer | trans -1,4-isoprene | ||||||
| Molecular formula of the repeating unit | C 5 H 8 | ||||||
| Molar mass of the repeating unit | 68.12 g mol −1 | ||||||
| Type of polymer |
Thermoplastic , plastomer |
||||||
| properties | |||||||
| Physical state |
firmly |
||||||
| density |
0.96-0.99 g · cm −3 ; air-free over 1.00 g cm −3 |
||||||
| Glass temperature |
38 ° C |
||||||
| solubility |
insoluble in water, stringy and sticky in boiling water; partly soluble in alcohol and ether; soluble in chloroform, benzene, toluene, petroleum, turpentine oil |
||||||
| Chemical resistance |
Hydrochloric acid, hydrofluoric acid |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
The (or that) gutta-percha or gutta is a rubber-like , rubber-like material from the dried, coagulated latex of different Sapotengewächsen ((Sapotaceae) Payena spp. And Palaquium spp. And other), as well as spindle bushes ( Euonymus spp.), Originally came they mainly come from the gutta-percha tree ( Palaquium gutta ).
The name is derived from Malay , get (t) ah = rubber (resin), sticky exudate, juice (milky sap) and pertja, percha = name of the tree or piece, rag, strips of cloth and also as the name for the island of Sumatra ; So rubber (resin), sap (milky sap) of the percha tree or Sumatra rubber and piece of rubber rag.
properties
Raw gutta-percha is brown to gray-brown, the raw material contains some sand, small pieces of wood and bark as impurities. It is fibrous, flaky, almost woody and easy to cut - in contrast to rubber - and flexible, but not elastic, a bit greasy with a leathery odor. After cleaning it then becomes plastic. It consists of approx. 50–75% gutta ( polyterpenes ), approx. 10–48% resins (Fluavil, Alban, Albanan), salts, nitrogen compounds and triterpenes ; Lupeol , esterified amyrine . Bassic acid occurs in some species of palaquium . It is then softened and cleaned with solvents as well as bleached, it is then white, solid and crystalline and almost odorless.
Pure gutta-percha is chemically similar to natural rubber, but in contrast to this, it is not composed of cis -, but of trans -configured 1,4- polyisoprene , with a far lower molar mass , as the molecule consists of far fewer repeat units. It is biocompatible and inert as well as optically anisotropic, i. H. birefringent and chemically much more resistant to aggressive media, in contrast to rubber. It can also be vulcanized , but this is usually not done. Their thermoplastic behavior (softening temperature 70–90 ° C) allows shaping processing without vulcanization.
At room temperature it is harder and not as elastic, but becomes soft and kneadable at approx. 48–60 ° C. The degree of polymerization (the number of monomeric units in a macromolecule) is around 1500, while that of natural rubber is around 8000-30,000.
At room temperature, the solid gutta-percha oxidizes quickly and becomes brittle, so it must be stored under water or enclosed airtight.
Similar products are the balata from the balata tree ( Manilkara bidentata ) and the chicle , which is obtained from different Manilkara species. The guayule rubber from the guayule ( Parthenium argentatum ) is also used to a greater extent, here the polyisoprenes are cis -configured.
to form
Gutta-percha can come in three forms; in two ordered ( crystalline ) alpha and beta as well as one disordered ( amorphous ). The crystalline alpha and beta forms differ in a different repeating unit in the polymer chain; one monomer ( beta ), two monomers ( alpha ), as well as the single bond configuration, which have two forms, in turn different crystallographic structures, the variations of which are reflected in volume changes that are induced in gutta-percha by heating and cooling.
Most commercial gutta-percha exists in beta form. The alpha form occurs in raw milky juice. When the natural alpha form is heated above 65ºC it becomes amorphous and melts. When this amorphous material is cooled extremely slowly (0.5 ° C per hour), the alpha form recrystallizes . On the other hand, when the amorphous melt is routinely cooled, the beta form recrystallizes . Most commercial gutta-percha, including dental gutta-percha, exist in this form. When the beta form is heated again, the polymer becomes amorphous at 56 ° C, 9 degrees lower than the melting point for the alpha form. It is therefore evident that the factor which determines the melting point of alpha and beta gutta-percha is the rate of cooling, which in turn controls the extent and character of crystallinity in the solidified material. The beta form converts to the alpha form at 42–49 ° C , which then becomes amorphous again at 53–56 ° C.
Applications
Dentistry
In dentistry , gutta-percha is mainly used for root canal treatments . The "gutta-percha points" used to fill the canals contain a high percentage of gutta-percha in addition to a number of other components. Even for temporary fillings, gutta-percha is sometimes still used today, e.g. B. to bridge the time between the preparation and the integration of an inlay . It has the advantage over synthetic temporary materials that it can be removed in one piece.
art
Gutta is also used in silk scarf painting as a separating agent to achieve contours. With this contour technique called Guttatechnik , the fabric is not colored where the release agent was applied. What remains is a colorless line or area.
Electroplating
In the second half of the 19th century, galvanoplastic methods were used to copy historical metalwork in small editions or to transfer small sculptures by artists into metal . Impressions with the help of the heated, elastic gutta-percha can also be easily taken from fully rounded bodies or strongly undercut reliefs. Made electrically conductive with graphite powder, the resulting shapes could be galvanically transferred into metal.
Sports
The golf ball was also a typical application for this material in the past. Such balls were called also Guttie, Gutty or Bramble .
Commodities
Gutta-percha buckets were very common in the chemical industry before more modern materials became available. Especially in dynamite factories Guttaperchaeimer were transporting smaller explosive oil - batches used and are often seen on older pictures. Gutta-percha was also used as an ingredient in chewing gum .
Electrical cable insulation
Because of its good insulating properties , the polyterpene was used for sheathing electrical cables from the middle of the 19th century . Material tests in 1846 and the invention of the extrusion press by Werner Siemens led to the establishment of the Telegraphen Bau-Anstalt von Siemens & Halske in 1847 . Such cables made intercontinental telegraphy possible in particular by laying the submarine cables . As an insulation material for electrical cables, gutta-percha has been completely replaced by various types of plastics .
literature
- Eugen Obach: The gutta-percha. Steinkopff & Springer, Dresden-Blasewitz 1899. Digitized edition of the University and State Library Düsseldorf .
- John Tully: A Victorian Ecological Disaster: Imperialism, the Telegraph, and Gutta-Percha. In: Journal of World History. Volume 20 (4), (2009), pp. 559-579.
- Handheld electrical telecommunications dictionary . Volume 2, 2nd edition, pp. 762-763.
Web links
- Meyer's Large Conversation Lexicon. Volume 8, Leipzig 1907, pp. 551–553: Gutta Percha at Zeno.org .
Individual evidence
- ^ Albert Gossauer: Structure and reactivity of biomolecules , Verlag Helvetica Chimica Acta, Zurich, 2006, p. 130, ISBN 978-3-906390-29-1 .
- ^ Hans G. Hirschberg: Handbook of process engineering and plant construction. Chemistry, technology and business administration. Springer, 1999, ISBN 978-3-540-60623-9 .
- ↑ a b c d e G. Frerichs, G. Arends, H. Zörnig: Hagers Handbook of Pharmaceutical Practice. First volume: A – I , Springer, 1949, ISBN 978-3-642-49473-4 (reprint), p. 1413.
- ↑ Hans-Georg Elias: Macromolecules. Volume 4: Applications of Polymers , 6th Edition, Wiley, 2003, ISBN 3-527-29962-9 , p. 265.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ Henry Yule , AC Burnell : Hobson-Jobson : The Definitive Glossary of British India. New Edition, Murray, 1903, p. 404 f, Oxford Univ. Press, 2013, ISBN 978-0-19-960113-4 (Reprint), p. 252.
- ↑ Rogers McVaugh, Harley Harris Bartlett: The Asa Gray Bulletin. New Series, Vol. II, No. I, 1953, p. 154, online at biodiversitylibrary.org, accessed January 20, 2018.
- ↑ Heinz. A. Hoppe: Drug science. Volume 1: Angiosperms , 8th edition, De Gruyter, 1975, ISBN 3-11-003849-8 , p. 781.
- ↑ R. Hansel, O. Sticher, E. Steinegger: Pharmakognosie - Phytopharmazie. Volume 1, 6th edition, Springer, 1999, ISBN 978-3-662-09270-5 , p. 49.
- ^ Elsa Franke, Reinhard Lieberei, Christoph Reisdorff: Useful plants. 8th edition, Thieme, 2012, ISBN 978-3-13-530408-3 , p. 394.
- ^ A. Goodman, H. Schilder, W. Aldrich: The thermomechanical properties of gutta-percha. II. The history and molecular chemistry of gutta-percha. In: Oral Surg. Oral Med. Oral Pathol. 37 (6), 1974, pp. 954-61, doi : 10.1016 / 0030-4220 (74) 90448-4 , online (PDF; 822 kB), on endoexperience.com, accessed on January 18, 2018.
- ^ A. Goodman, H. Schilder, W. Aldrich: The thermomechanical properties of gutta-percha. III. Determination of phase transition temperatures for gutta-percha. In: Oral Surg. Oral Med. Oral Pathol. 38 (1), 1974, pp. 109-114, doi : 10.1016 / 0030-4220 (74) 90321-1 .
- ^ Alfred Löhr: Electroplating in the Bremen silver goods industry. In: Jörn Christiansen (Ed.): Bremen is getting bright, 100 years of living and working with electricity. Hauschild, Bremen 1993, ISBN 978-3-926598-95-0 , pp. 271-273.
- ↑ Silvia Glaser: Gutta-percha. In: Historical plastics in the Germanic National Museum. Verlag des Germanisches Nationalmuseums, Nuremberg 2008, ISBN 978-3-936688-37-5 , p. 7.
