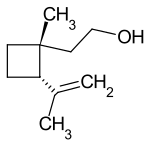
Grandisol
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| General | |||||||||||||
| Surname | Grandisol | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 10 H 18 O | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 154.25 g mol −1 | ||||||||||||
| boiling point |
50-60 ° C (133 Pa) |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Grandisol is a natural product with the empirical formula C 10 H 18 O. It is a monoterpene that contains a cyclobutane ring , an alcohol group, a double bond and two stereocenters.
Grandisol is a pheromone that is primarily important as an attractant for the cotton boll beetle Anthonomus grandis , hence the name. It is also a pheromone for other insects. The cotton boll beetle is a crop pest that can cause significant economic damage.
synthesis
Grandisol was first used in 1969 by J. Tumlinson et al. isolated, identified, and synthesized at Mississippi State University .
The current synthesis with the highest yield was published in January 2010 by chemists at Furman University . Although enantioselective syntheses are known, racemic grandisole has been shown to be just as effective in attracting boll weevils as the pure enantiomer . The racemate of this pheromone can be useful for plant protection. The synthesis of enantioselective grandisol could still be useful, because there is potential for the pheromone to be used as a medicinal substance.
literature
- Y.-S. Kwak, BS Jeong: Arch Pharm. Res. (2011), 34, 1399, doi: 10.1007 / s12272-011-0900-y
- D. Kim, Y.-S. Kwak, KJ Shin: A stereospecific synthesis of (±) -grandisol via an intramolecular lactone enolate alkylation: A remarkable regiodivergence in C- vs O-alkylation. In: Tetrahedron Lett. Volume 35, 1994, pp. 9211-9212. doi: 10.1016 / 0040-4039 (94) 88468-4 .
- K. Langer, J. Mattay: Stereoselective Intramolecular Copper (I) -Catalyzed [2 + 2] - Photocycloadditions. Enantioselective Synthesis of (+) - and (-) - Grandisol. In: J. Org. Chem. Volume 60, 1995, pp. 7256-7266, doi: 10.1021 / jo00127a034 .
- T. Martin, CM Rodríguez: A new approach to functionalizes cyclobutanes: Stereoselctive synthesis of the enantiomers of grandisol and fragranol. In: Tetrahedron Asymmetry. Volume 6, 1995, pp. 1151-1164. doi: 10.1016 / 0957-4166 (95) 00141-B .
Web links
Individual evidence
- ↑ a b Burkhard Fugmann: RÖMPP Lexikon Naturstoffe . Georg Thieme Verlag, 2014, ISBN 978-3-13-179541-0 , p. 2298 ( limited preview in Google Book Search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Thomas JA Graham, Erin E. Gray, James M. Burgess, Brian C. Goess: An Efficient Synthesis of (±) -Grandisol Featuring 1,5-Enyne Metathesis . In: J. Org. Chem. Band 75 , no. 1 , January 2010, p. 226-228 , doi : 10.1021 / jo9020375 , PMID 19957923 .
- ↑ B. Hibbard, F. Webster: Enantiomeric composition of grandisol and grandisl produced by Pissodes strobi and P. nemorensis and their electroantennogram response to pure enantiomers . In: J. Chem. Ecol. tape 19 , no. 10 , Oct. 1993, p 2129-2141 , doi : 10.1007 / BF00979652 .