Diterpenes
Diterpenes are made up of four isoprene units (2-methylbutadiene). The approximately 3000 known, natural diterpenes are thus compounds consisting of 20 carbon atoms that can be assigned to the terpene group of substances. They can be divided into open-chain and cyclic compounds, which can be traced back to phytane (2,6,10,14-tetramethylhexadecane).
Phytans
| Connection example | Use / occurrence |
|---|---|
|
(3 R , 7 R , 11 R ) -phytane |
Component in various sediments, for example in oil shale |
|
(-) - (3 R , 7 R , 11 R ) -phytanoic acid |
Trace component in butter |
Phytane can be found in the form of (3 R , 7 R , 11 R ) -phytane in sedimentary rocks, oil shale and the human liver, among other things. The phytanic is also present in the oil shale and can be detected in trace amounts in butterfat. Phytanic acid plays a role in Refsum's syndrome . The acid is deposited here due to a disruption of the lipoid metabolism in the organ fat, in the fatty tissue of the muscles and in the urine of the person concerned. Winfried Kahlke first brought phytanic acid into connection with Refsum's syndrome in 1963 as part of an organ examination of a deceased child.
Cyclophytanes
While 1,6-cyclophytanes are rare, 10,15-cyclophytanes are more common in nature:
| Connection example | Use / occurrence |
|---|---|
|
11- cis -retinal (top) and all-trans -retinal (bottom) |
11- cis -retinal occurs in rhodopsin in the retina of the eye |
An example of a 10,15-cyclophytane is 11- cis - retinal , which occurs in the form of vitamin A aldehyde . Together with the protein opsin as a component of rhodopsin in the rods of the retina of the eye, it plays a crucial role in photoreception . The capture of a photon triggers the conversion of 11- cis -retinal into the more stable all-trans -retinal and a number of other chemical reactions in the rod, more precisely in rhodopsin . Metarhodopsin II, an intermediate product of these chemical reactions, leads to the deactivation of cGMP and thus the closure of the sodium-calcium channels (which are open without incident light); it is referred to as hyperpolarization . An enzymatic reaction deactivates the metarhodopsin so that the conversion of all-trans -retinal into the more light-sensitive 11- cis -retinal can take place.
Bicyclophytanes
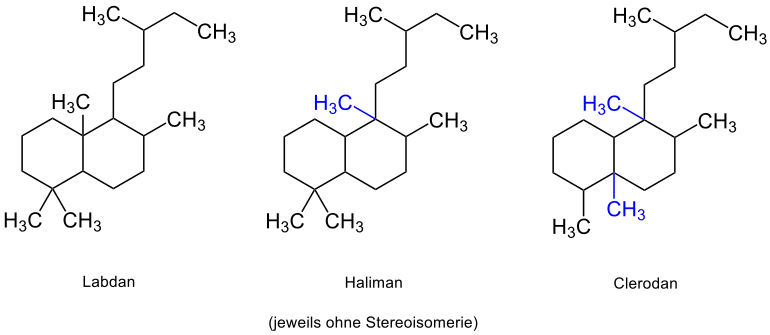
The Bycyclophytane can be traced back to the basic structures of Labdan , Haliman and Clerodan . The approximately 500 known labdanes are 6,11-10,15-cyclophytanes, which are plant-based compounds that are important in the field of fragrances. The structures are based on the bicyclic decalin ( marked in blue ):
| Connection example | Use / occurrence |
|---|---|
|
(-) - Sclareol, a labdan |
Natural occurrence of (-) - Sclareol in Salvia sclarea , the clary sage |
The Labdane owe their name to the rock rose bush Cistus labdaniferus . Raw materials such as labdanum resin for the perfume industry are obtained from the branches of this shrub. Another perfumery raw material is (-) - sclareol , which functions as a starting material in the partial synthesis of the fragrance Ambrox . Ambrox is the most important ingredient in ambergris , a waxy mass from the intestines of the sperm whale.
Rearranged bicyclophytans
The basic structure of Haliman or Clerodan is obtained through rearrangement of one or two methyl groups in labdane (rearranged methyl groups are each marked in blue ):
| Connection example | Use / occurrence |
|---|---|
|
Agelasin-C, a Haliman |
Inhibitory effect on the sodium-potassium-ATPase shown below: Sodium and potassium ions are transported through the membrane under normal conditions with consumption of ATP. |
|
Agelasin-B, a clerodan |
The Halimans owe their name to Cistaceaen plants such as Halimium Umbellatum . Various derivatives are also found, for example, in the form of agelasins in the extracts of various sea sponges, such as the orange Okinawan sea sponge, Agelas sp . The Halimander derivatives agelasin-C and -D have an inhibitory effect on the cell membrane enzyme sodium-potassium-ATPase . An inhibition of sodium-potassium-ATPase is also caused therapeutically by cardiac glycosides. The reversibly controlled transport inhibition of sodium and potassium ions ensures an increased concentration of sodium and consequently calcium ions within the cell, which results in a positive inotropic effect. An overdose of sodium-potassium-ATPase-inhibiting substances leads to an uncontrollable release of calcium ions and post-depolarization of the cell, which leads to arrhythmias of the heart muscle. The clerodan derivative Agelasin-B, which can also be isolated from Agelas sp and the sponges Agelas nakamurai and Agelas cf. mauritiana , has a similar inhibitory effect . Investigations suggest an anti-inflammatory and possibly cancer-preventive mode of action of agelasin-B at a concentration of 20 µM. With the correct dosage, the agelasins generally have an antispasmodic and antibacterial effect.
Tricyclophytanes
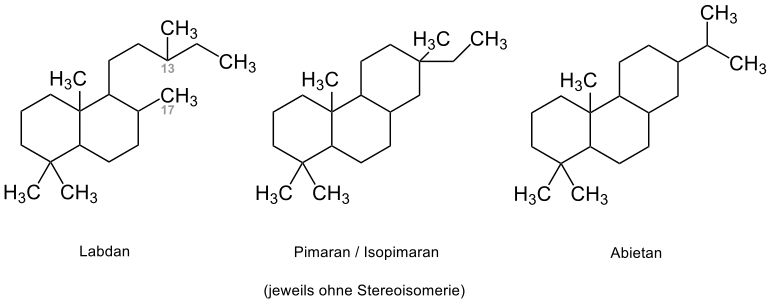
The pimarans and isopimarans can be derived from a cyclization at C-13 and C-17 of the labdan, whereby these two substances can be distinguished by their configuration at C-13. Due to rearrangements, groups such as the rosans, toxic substances of the fungus Trichothecium roseum , the cassans, the starting material of the locally anesthetically effective alkaloids of cassainic acid, and the abietans are among the representatives of the tricyclophytans.
| Connection example | Use / occurrence |
|---|---|
|
12α-Hydroxy-8,15-isopimaradien-18-acid, an isopimaran |
Metasequoia glyptostroboides , a "living fossil" |
|
(-) - Abietic acid, an abietane |
Part of the rosin |
Components obtained from Metasequoia glyptostroboides , such as 12α-hydroxy-8,15-isopimaradien-18-acid, have a cytotoxic effect on various human tumor cell lines, such as the A-549 cell line . Metasequoia glyptostroboides was first discovered in 1946 by Dr. Hsen-Hsu Hu was discovered in Wan Hsien in China and is colloquially referred to as the "living fossil". The classification sequoia was introduced by Stephanus Endlicher in 1847 for sequoias and related species based on the Sequoyah tribe of the Cherokee Indians.
The abietic represents the essential representative of Abietane. The recovery can be made by distillation from the rosin done. Rosin is obtained by steam distillation from the balsamic resin of various types of pine, such as Pinus palustris , and can be used to test allergic reactions to certain fragrances. In the resin, abietic acid fulfills its function as a protective secret against insects and microbial infections. Technically, in addition to its use in paint components, abietic acid is used in the production of metal soaps and in lactic acid and butyric acid fermentation. Antimicrobial, cardiovascular, allergenic and antiallergenic uses are also reported.
Tetracyclophytanes
The tetracyclophytans can generally be derived from the pimaran. These include the Beyerans, Kaurane, Villanovane, Atisane, Gibberellane and Grayanotoxane. The basic skeletons of some of these representatives are listed below:
| Connection example | Use / occurrence |
|---|---|
|
(-) - Cafestol, a Kauran |
Arabica plant and fruits |
|
Gibberellic acid, a gibberellan |
Promotes the growth of young pea plants. |
|
Rhodojaponin III, a grayanotoxan |
Insecticidal effect on the Colorado potato beetle. |
The 1,500 known kaurans include, for example , the anti-inflammatory furanokauran (-) - cafestol obtained from Coffea arabica . Studies also show a connection between coffee consumption and a reduced risk of colorectal tumors .
The steviosides are kauran glycosides that have been isolated from the Stevia rebaudiana plant . Some representatives, e.g. B. Stevioside and the rebaudioside A, D and M, are more than 150 times as sweet as sucrose and are used as natural sweeteners.
There are 35 known gibberellans, which are phytohormonal substances that can influence plant growth or the formation of fruits. The growth is influenced by changing the ion transport from the root to the shoot.
Gibberellic acid has a positive effect on the absorption of potassium ions in the roots and the subsequent transport into the shoot and thus on the elongated growth of young pea plants.
The grayantoxanes form a group of toxic ingredients in Ericaceae plants. The grayanotoxan rhodojaponin III occurs in the Chinese rhododendron species Rhododendron molle and has an insecticide- inhibiting and insecticidal effect. Insecticidal effects have been demonstrated against the Colorado potato beetle Leptinotarsa decemlineata , originally native to America and represented on European soil since 1877, and the army worm species Spodoptera frugiperda .
Further representatives of the diterpenes
| category | Connection example | Use / occurrence |
|---|---|---|
| Cembrane |
Cembren A, a cembrane |
Acts as a pheromone on termites. |
| Cyclocembrane |
Casben, a casban |
Protects the Ricinus communis plant (Chimoio, Mozambique) from fungal attack. |
|
(+) - Daphnetoxin, a daphnan |
Found naturally in Daphne mezereum . |
Cembren A is one of the 100 known membranes , acts as a pheromone on termites and occurs in the essential oils of higher plants. It is also an ingredient in frankincense.
Taxanes occur only in the conifer genus Taxus and are used as cytostatics.
Casbans are generally rare substances, but in higher plants such as the Euphorbiaceaen family Ricinus communis they act as protection against fungal attack, like casben.
Phorbols occur in Euphorbiaceen and Thmyleaceen. Lipophilic derivatives, especially esters with long-chain fatty acids such as phorbol-12-myristate-13-acetate or orthoesters with benzoic acid such as daphnetoxin, which is contained in the Daphne mezereum daphne , cause severe skin irritation and allergic reactions in humans when they come into contact with the skin and mucous membranes . Daphnetoxin can only be found in plants of the Thymelaeaceae and has mitochondrial toxic properties and a possibly cancer-inhibiting effect.
Further subgroups of the diterpenes are the prenylsesquiterpenes, in which the basic skeleton of a sesquiterpene is supplemented by an isoprenyl residue, and the ginkgolides , ingredients of the ginkgo tree.
Individual evidence
- ↑ a b Eberhard Breitmaier: Terpenes: flavors, fragrances, pharmaceuticals, pheromones. 2nd edition, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, 2005, p. 2, doi: 10.1002 / 9783527623693 .
- ↑ a b c d e f g Eberhard Breitmaier: Terpenes: flavors, fragrances, pharmaceuticals, pheromones. 2nd edition, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, 2005, p. 51, doi: 10.1002 / 9783527623693 .
- ↑ a b c d Ernst Klenk, Winfried Kahlke: About the occurrence of 3.7.11.15-tetramethyl-hexadecanoic acid (phytanic acid) in the cholesterol esters and other lipoid fractions of the organs in a case of illness of unknown origin (suspected Heredopathia atactica polyneuritiformis [Refsum syndrome]) . In: Hoppe-Seyler's journal for physiological chemistry. , Volume 333, 1963, pp. 133-139, doi: 10.1515 / bchm2.1963.333.1.133 .
- ↑ a b c d e f Eberhard Breitmaier: Terpenes: flavors, fragrances, pharmaceuticals, pheromones. 2nd edition, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, 2005, p. 52, doi: 10.1002 / 9783527623693 .
- ↑ a b c Eberhard Betz, Dieter Mecke, Klaus Reutter, Horst Ritter: Mörike / Betz / Mergenthaler. Human biology. 15th corrected edition, Quelle & Meyer Verlag GmbH & Co., Wiebelsheim, 2001, ISBN 3-494-01297-0 , pp. 564-565.
- ^ A b George P. Rédei: Encyclopedia of Genetics, Genomics, Proteomics, and Informatics. Volume 2 MZ, 3rd edition, Springer, 2008, ISBN 978-1-4020-6754-9 , p. 1703.
- ↑ a b c d e f Eberhard Breitmaier: Terpenes: flavors, fragrances, pharmaceuticals, pheromones. 2nd edition, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, 2005, p. 53, doi: 10.1002 / 9783527623693 .
- ↑ Eberhard Breitmaier: Terpenes: flavors, fragrances, pharmaceuticals, pheromones. 2nd edition, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, 2005, p. 54, doi: 10.1002 / 9783527623693 .
- ↑ a b Bernd Schäfer: Ambrox®. Irresistible fragrance. In: Chemistry in Our Time . No. 45, 2011, pp. 374–388, doi: 10.1002 / ciuz.201100557 .
- ↑ a b c d Hideshi Nakamura, Houming Wu, Yasushi Ohizumi, Yoshimasa Hirata: Agelasine-A, -B, -C and -D, novel bicyclic diterpenoids with a 9-methyladeninium unit possessing inhibitory effects on na, K-atpase from the okinawa sea sponge Agelas sp. In: Tetrahedron Letters. Volume 25, No. 28, 1984, pp. 2989-2992, doi: 10.1016 / S0040-4039 (01) 81345-9 .
- ↑ a b c E. K. Ogurtsova, TN Makarieva, PS Dmitrenok, VA Denisenko, VB Krasokhin, AS Kuz'mich, SN Fedorov: ISOLATION OF AGELASIN B FROM THE MARINE FUNGUS Agelas cf. mauritiana. In: Chemistry of Natural Compounds. Volume 51, No. 1, January, 2015, pp. 189-191, doi: 10.1007 / s10600-015-1241-8 .
- ↑ Eberhard Breitmaier: Terpenes: flavors, fragrances, pharmaceuticals, pheromones. 2nd edition, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, 2005, p. 55, doi: 10.1002 / 9783527623693 .
- ↑ a b c Hans Marquardt, Siegfried G. Schäfer (Ed.): Textbook of Toxicology. 2nd edition, Wissenschaftliche Verlagsgesellschaft mbH Stuttgart, 2004, ISBN 3-8047-1777-2 , pp. 558-559.
- ↑ a b Eberhard Breitmaier: Terpenes: flavors, fragrances, pharmaceuticals, pheromones. 2nd edition, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, 2005, pp. 56-58, doi: 10.1002 / 9783527623693 .
- ↑ a b c d Liao-Bin Dong, Juan He, Yuan-Yuan Wang, Xing-De Wu, Xu Deng, Zheng-Hong Pan, Gang Xu, Li-Yan Peng, Yu Zhao, Yan Li, Xun Gong, Qin- Shi Zhao: Terpenoids and Norlignans from Metasequoia glyptostroboides. In: J. Nat. Prod. 2011, Volume 74, No. 2, pp. 234-239, doi: 10.1021 / np100694k
- ↑ a b c d e Wolfgang Steglich, Burkhard Fugmann, Susanne Lang-Fugmann (eds.): RÖMPP Lexikon. Natural substances. 10th edition, Georg Thieme Verlag, Stuttgart, 1997, ISBN 3-13-749901-1 , p. 1.
- ↑ Christoph Funk, Rodney Croteau: Diterpenoid Resin Acid Biosynthesis in Conifers: Characterization of Two Cytochrome P450-Dependent Monooxygenases and an Aldehyde Dehydrogenase Involved in Abietic Acid Biosynthesis. In: Archives of Biochemistry and Biophysics. 1994, Volume 308, No. 1, pp. 258-266, doi: 10.1006 / abbi.1994.1036
- ↑ Eberhard Breitmaier: Terpenes: flavors, fragrances, pharmaceuticals, pheromones. 2nd edition, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, 2005, p. 61, doi: 10.1002 / 9783527623693 .
- ^ A b Edmund H. Fulling: Metasequoia. Fossil and Living. In: The Botanical Review. 1976, Vol. 42, No. 3, pp. 215-219, JSTOR 4353902 .
- ↑ Andrea Nardelli, An Carbonez, Jacques Drieghe, An Goossens: Results of patch testing with fragrance mix 1, fragrance mix 2, and their ingredients, and Myroxylon pereirae and colophonium, over a 21-year period. In: Contact Dermatitis. May 2013, Volume 68, No. 5, pp. 307-313, doi: 10.1111 / cod.12056
- ↑ Johanna Brared Christensson, Klaus E. Andersen, Magnus Bruze, Jeanne D. Johansen, Begona Garcia-Bravo, Ana Giménez-Arnau, Chee-Leok Goh, Rosemary Nixon, Ian R. White: An international multicentre study on the allergenic activity of air-oxidized R-limonene. In: Contact Dermatitis. April 2013, Volume 68, No. 4, pp. 214-223, doi: 10.1111 / cod.12036
- ↑ Wolfgang Steglich, Burkhard Fugmann, Susanne Lang-Fugmann (eds.): RÖMPP Lexikon. Natural substances. 10th edition, Georg Thieme Verlag, Stuttgart, 1997, ISBN 3-13-749901-1 , p. 638.
- ^ A b Arturo San Feliciano, Marina Gordaliza, Miguel A. Salinero, José M. Miguel del Corral: Abietane Acids: Sources, Biological Activities, and Therapeutic Uses. In: Planta Med. 1993, Vol. 59 No. 6, pp. 485-490, doi: 10.1055 / s-2006-959744
- ↑ a b c d e f g h i Eberhard Breitmaier: Terpenes: flavors, fragrances, pharmaceuticals, pheromones. 2nd edition, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, 2005, pp. 63-66, doi: 10.1002 / 9783527623693 .
- ↑ a b c U. Lüttge, K. Bauer, D. Köhler: Early effects of gibberellic acid on membrane transport in young pea plants. In: Biochimica et Biophysica Acta. (BBA) - Biomembranes. Volume 150, No. 3, 1968, pp. 452-459, doi: 10.1016 / 0005-2736 (68) 90144-2 .
- ↑ a b James A. Klocke, Mei-Ying Hu, Shin-Foon Chiu, Isao Kubo: Grayanoid diterpene insect antifeedants and insecticides from Rhododendron molle. In: Phytochemistry. Volume 30, No. 6, 1991, pp. 1797-1800, doi: 10.1016 / 0031-9422 (91) 85015-R
- ↑ C. Cavin, D. Holzhaeuser, G. Scharf, A. Constable, WW Huber, B. Schilter: Cafestol and kahweol, two coffee specific diterpenes with anticarcinogenic activity. In: Food and Chemical Toxicology. Volume 40, No. 8, 2002, pp. 1155-1163, doi: 10.1016 / S0278-6915 (02) 00029-7 .
- ↑ Mei-Ying Hu, James A. Klocke, Shin-Foon Chiu, Isao Kubo: Response of Five Insect Species to a Botanical Insecticide, Rhodojaponin III. In: J Econ Entomol. Volume 86, No. 3, 1993, pp. 706-711, doi: 10.1093 / jee / 86.3.706 .
- ^ Heinz Freude, Karl Wilhelm Harde, Gustav Adolf Lohse, Wilhelm Lucht: Die Käfer Mitteleuropas: Cerambycidae, Chrysomelidae. 9. Goecke & Evers Verlag, Krefeld, 1966, ISBN 3-87263-018-0 , p. 151.
- ↑ a b c d e f g h i Eberhard Breitmaier: Terpenes: flavors, fragrances, pharmaceuticals, pheromones. 2nd edition, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, 2005, pp. 67-75, doi: 10.1002 / 9783527623693 .
- ↑ Wolfgang Steglich , Burkhard Fugmann, Susanne Lang-Fugmann (eds.): RÖMPP Lexikon. Natural substances. 10th edition, Georg Thieme Verlag, Stuttgart, 1997, ISBN 3-13-749901-1 , p. 119.
- ↑ Cátia V. Diogo, Luís Félix, Sérgio Vilela, Ana Burgeiro, Inês A. Barbosa, Maria JM Carvalho, Paulo J. Oliveira, Francisco P. Peixoto: Mitochondrial toxicity of the phyotochemicals daphnetoxin and daphnoretin - Relevance for possible anti-cancer application . In: Toxicology in Vitro. Volume 23, No. 5, 2009, pp. 772-779, doi: 10.1016 / j.tiv.2009.04.002
- ↑ Eberhard Breitmaier: Terpenes: flavors, fragrances, pharmaceuticals, pheromones. 2nd edition, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, 2005, pp. 76-80, doi: 10.1002 / 9783527623693 .