Phorbol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
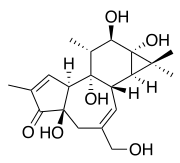
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Phorbol | |||||||||||||||
| other names |
1,1a, 1b, 4,4a, 7a, 7b, 8,9,9a-decahydro-4a, 7b, 9,9a-tetrahydroxy-3- (hydroxymethyl) -1,1,6,8-tetramethyl-5 H -cyclopropa [3,4] benz [1,2- e ] azulen-5-one |
|||||||||||||||
| Molecular formula | C 20 H 28 O 6 | |||||||||||||||
| Brief description |
white solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 364.44 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
250–251 ° C (decomposition) |
|||||||||||||||
| boiling point |
572 ° C (760 mmHg) |
|||||||||||||||
| solubility |
Soluble in ethanol at 50 mg / ml as a colorless to slightly yellowish solution |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Phorbol is a natural substance that is produced by numerous plants, especially the milkweed family and the daphne family . It was first extracted from croton oil in 1934 . Phorbol itself is a relatively harmless alcohol, but its esters in particular are toxic and responsible for numerous poisonings.
Chemical structure and synthesis
Phorbol is a tetracyclic diterpene and chemically similar to other toxins of the milkweed family such as ingenol mebutate and the daphne family such as daphnetoxin and mezerein . As with many natural products, total synthesis is challenging but possible. The successful total synthesis makes it possible to study chemically similar compounds that are not available in nature in the laboratory and to evaluate their potential as active ingredients.
Natural occurrence and extraction
Phorbol was first extracted from croton oil, which had attracted some attention due to its medicinal uses and toxicity. The toxicity of croton oil is caused by eighteen slightly different diesters of phorbol, which can be extracted by washing with methanol and subsequent purification.

Phorbol esters make a substantial contribution to the toxicity of the manchinel tree and its fruits, the manzanilla de la muerte (apple of death).
Symptoms of intoxication
Phorbol esters such as phorbol 12-myristate 13-acetate mimic 1,2-diacylglycerols , an activator of protein kinase C . This protein kinase is a key component of cellular signal transduction , which is massively disrupted as a result.
Plants with higher contents of phorbol esters are problematic feedstuffs in the animal fattening of herbivores. Poisoning in humans is known from honey or the consumption of phorbol-containing parts of plants.
Basic research
Numerous esters of phorbol have biologically remarkable properties. In the basic research they are for induction of tumors and to enhance the secretion of neurotransmitters used.
Individual evidence
- ↑ a b c d Data sheet Phorbol from Sigma-Aldrich , accessed on March 1, 2017 ( PDF ).
- ↑ a b c d data sheet Phorbol at Alfa Chemistry, accessed March 1, 2017.
- ↑ G. Goel, HP Makkar, G. Francis, K. Becker: Phorbol esters: structure, biological activity, and toxicity in animals. In: International Journal of Toxicology . Volume 26, Number 4, 2007 Jul-Aug, pp. 279-288; doi: 10.1080 / 10915810701464641 , PMID 17661218 (Review).
- ↑ B. Flaschträger, R. Wolffersdorff: About the poison of croton oil. In: Helvetica Chimica Acta . Volume 17, Number 1, 1934, pp. 1444-1452; doi: 10.1002 / hlca.193401701179 .
- ↑ S. Kawamura, H. Chu, J. Felding, PS Baran: Nineteen-step total synthesis of (+) - phorbol. In: Nature . Volume 532, Number 7597, April 2016, pp. 90-93; doi: 10.1038 / nature17153 , PMID 27007853 , PMC 4833603 (free full text).
- ↑ W. Blaschek, R. Hansel, K. Keller: Hagers Handbook of Pharmaceutical Practice. Volume 2: Drugs A – K , 5th edition, Springer, 1998, ISBN 978-3-642-63794-0 , pp. 471–476, limited preview in Google book search.
- ↑ E. Hecker: Phorbol esters from croton oil. Chemical nature and biological activities. In: The natural sciences . Vol. 54, Number 11, June 1967, pp. 282-284; PMID 5589922 .
- ↑ JG Meyer-Bertenrath: 150 Years of Croton Oil Research. In: Experientia . Volume 25, Number 1, January 1969, pp. 1-5; PMID 4885798 .
- ↑ B. Flaschträger, G. Wigner: About the toxin of croton oil. V. The extraction of croton resin, thin oil and phorbol from croton oil by alcoholysis. In: Helvetica Chimica Acta . Volume 25, Number 3, 1942, pp. 569-581; doi: 10.1002 / hlca.19420250315 .
- ^ W. Adolf, E. Hecker: On the active principles of the spurge family, X. Skin irritants, cocarcinogens, and cryptic cocarcinogens from the latex of the manchineel tree. In: Journal of Natural Products . Volume 47, Number 3, 1984 May-Jun, pp. 482-496; PMID 6481361 .
- ↑ a b N. H. Strickland: Eating a manchineel "beach apple" In: BMJ . Volume 321, Number 7258, August 2000, p. 428; PMID 10938053 , PMC 1127797 (free full text).
- ↑ a b P. M. Blumberg: Protein kinase C as the receptor for the phorbol ester tumor promoters: sixth Rhoads memorial award lecture. In: Cancer Research . Volume 48, Number 1, January 1988, pp. 1-8; PMID 3275491 (Review).
- ↑ a b E. M. Silinsky, TJ Searl: Phorbol esters and neurotransmitter release: more than just protein kinase C? In: British Journal of Pharmacology . Volume 138, Number 7, April 2003, pp. 1191-1201; doi: 10.1038 / sj.bjp.0705213 , PMID 12711617 , PMC 1573789 (free full text) (review).
- ↑ G. Goel, HP Makkar, G. Francis, K. Becker: Phorbol esters: structure, biological activity, and toxicity in animals. In: International Journal of Toxicology . (2007), Volume 26, Issue 4, pp. 279-288; PMID 17661218 .
- ↑ S. Sosath, HH Ott, E. Hecker: Irritant principles of the spurge family (Euphorbiaceae). XIII. Oligocyclic and macrocyclic diterpene esters from latices of some Euphorbia species utilized as source plants of honey. In: Journal of Natural Products . Volume 51, Number 6, 1988 Nov-Dec, pp. 1062-1074; PMID 3236005 .

