Ambrox
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||
| Structural formula of (-) - Ambrox | ||||||||||
| General | ||||||||||
| Surname | Ambrox | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 16 H 28 O | |||||||||
| Brief description |
white solid with a characteristic odor |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 236.39 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| density |
0.939 g · cm −3 [(-) - Ambrox] |
|||||||||
| Melting point |
73–75 ° C [760 mm Hg, (-) - Ambrox] |
|||||||||
| boiling point |
120 ° C [1.4 mm Hg, (-) - Ambrox] |
|||||||||
| solubility |
|
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Ambrox (also Ambroxan ) is a chemical compound from the class of tetranlabdane oxides . The source used in the past was ambergris , which is found in the intestines of whales . Ambrox is considered to be one of the most valuable animal fragrances next to civet and musk .
Occurrence
Ambrox produced by the reaction of ambrein (main component of ambergris, a waxy substance from the digestive tract of sperm whales) with atmospheric oxygen. Ambrox is also found in small amounts in the following sources:
- Absolue of tobacco ( Nicotiana tabacum )
- in the oil of clary sage ( Salvia sclarea )
- in the rock rose ( Cistus labdaniferus )
- and in the black cypress ( Cupressus sempervirens )
Extraction and presentation
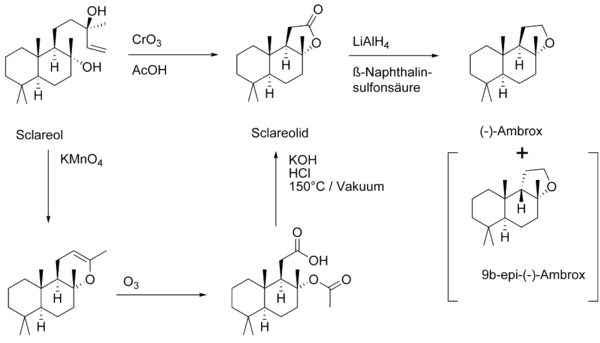
Ambrein is no longer used to obtain Ambrox, it is replaced as a raw material by Sclareol from Salvia sclarea . An alternative Ambrox source was found when the company Firmenich published the first partial synthesis in 1950 . The key step in technical synthesis is the oxidative degradation of the side chain with chromium trioxide CrO 3 . The subsequent reduction of the lactone with lithium aluminum hydride leads to the diol, which cyclizes in the presence of acids to the desired ether. The thermodynamically more stable 9b- epi -Ambrox can arise as a by-product . The reaction can be carried out on an industrial scale with a yield of 54%.
After the patent expired in the early 1980s, further, technically feasible synthesis routes were published. A degradation reaction of sclareol using sodium periodate (instead of potassium permanganate ) has been published in the working group of Sir Derek HR Barton and a patent has been applied for.
Although the synthesis steps can be carried out relatively easily and safely, the major disadvantage of these syntheses is the fluctuating supply situation and thus the strongly varying prices of sclareol.
As an alternative to Sclareol, (+) - cis - Abienol can also be used to make Ambrox. (+) - cis-Abienol is obtained from Canada balsam .
By means of ozonolysis of (+) - cis -abienol and subsequent reductive work-up, a diol is obtained which can be cyclized to Ambrox with tosyl chloride in pyridine with excellent yields. In addition to this particularly clever degradation reaction from a synthetic point of view, Ambrox can also be obtained from a number of other natural substances [(-) - Drimenol , (-) - Thujone or (+) - Carvone ].
Use and properties
Ambrox is a fragrance that has been known since ancient times and is used in perfume manufacture.
The different Ambrox stereoisomers have a comparable odor, but differ in their odor thresholds .
| isomer | Odor threshold in ppb |
|---|---|
| (-) - Ambrox | 0.3 |
| 3a- epi - (-) - Ambrox | 34 |
| 9b- epi - (-) - Ambrox | 0.15 |
| (+) - Ambrox | 2.4 |
The annual world production for Ambrox and analogues is just over 30 tons.
Trade names
- Ambro, Ambroxan, Amberlynx: (-) - enantiomer
- Cetalox: racemate
Individual evidence
- ↑ Lansdowne aromatics: Lanbroxyde (PDF; 46 kB), accessed on June 17, 2017.
- ↑ Entry on Ambrox at ChemBlink , accessed on January 9, 2013.
- ↑ a b c d Entry on Ambroxan at thegoodscentscompany.com, accessed on June 17, 2017.
- ↑ a b data sheet (-) - Ambroxide, ≥99% from Sigma-Aldrich , accessed on January 9, 2013 ( PDF ).
- ↑ G. Ohloff: The Fragrance of Ambergris. In: Ernst T. Theimer: Fragrance chemistry: the science of the sense of smell. Academic Press, New York, 1982, ISBN 0-12-685850-0 .
- ↑ a b B. Schäfer: Chemistry in our time . 2011, 45, 374-388; doi: 10.1002 / ciuz.201100557 .
- ↑ Patent US4970163 : Process for producing diol and lactone and microorganisms capable of the same. Filed September 28, 1989 , published November 13, 1990 , inventors: Mohamad I. Farbood, James A. Morris, Arthur E. Downey. ,
- ↑ G. Ohloff, W. Giersch, W. Pickenhagen, A. Furrer and B. Frei: Significance of the geminal dimethyl group in the odor principle of Ambrox®. In: Helv. Chim. Acta . 1985, 68, 2022-2029, doi: 10.1002 / hlca.19850680726 .
- ^ AF Barrero, EJ Alvarez-Manzaneda, J. Altarejos, S. Salido and JM Ramos: Synthesis of Ambrox® from (-) - sclareol and (+) - cis-abienol. In: Tetrahedron . 1993, 49; 45, 10405-10412, doi: 10.1016 / S0040-4020 (01) 80567-6 .
- ↑ H. Akita, M. Nozawa, A. Mitsuda and H. Ohsawa: A convenient synthesis of (+) - albicanol based on enzymatic function: Total syntheses of (+) - albicanyl acetate, (-) - albicanyl 3,4- dihydroxycinnamate, (-) - drimenol, (-) - drimenin and (-) - ambrox. In: Tetrahedron: Asymmetry . 2000, 11, pp. 1375-1388, doi: 10.1016 / s0957-4166 (00) 00076-8 .
- ↑ JP Kutney and C. Cirera: The chemistry of thujone. XX. New enantioselective syntheses of Ambrox and epi-Ambrox . In: Canadian Journal of Chemistry . 75 (8), 1997, p. 1136, doi : 10.1139 / v97-136 .
- ↑ AA Verstqen-Haaksma, HJ Swarts, BJM Jansen and A. de Groot: Total synthesis of (-) - Ambrox® from S - (+) - carvone. In: Tetrahedron. 1994, 50; 33, 10095-10106, doi: 10.1016 / S0040-4020 (01) 89625-3 .
- ↑ G. Fráter, YES Bajgrowicz, P. Kraft: Fragrance Chemistry. In: Tetrahedron. 1998, 54; 27, 7633-7703.
- ^ C. Allemann: Synthesis and Application of an Electronically Chiral Mimic of CpFe. Dissertation, No. 1369, Université de Friborg (Suisse), 2011, urn : nbn: ch: rero-002-101591 .