Nerolidol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
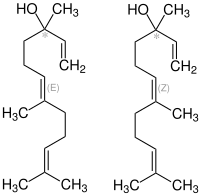
| trans -Nerolidol (left) and cis -Nerolidol (right) - in each case racemates | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Nerolidol | |||||||||||||||
| other names |
3,7,11-trimethyl-1,6,10-dodecatrien-3-ol |
|||||||||||||||
| Molecular formula | C 15 H 26 O | |||||||||||||||
| Brief description |
colorless to light yellow liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 222.37 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.88 g cm −3 |
|||||||||||||||
| boiling point | ||||||||||||||||
| solubility |
bad in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Nerolidol is one of the acyclic sesquiterpenes or rather one of the sesquiterpene alcohols. It is the main ingredient in Cabreuva oil . Nerolidol is an isomer of farnesol . Both alcohols contain three double bonds, whereby nerolidol differs from farnesol in the position of a double bond and the OH group.
Occurrence
Nerolidol is found in neroli , ginger , jasmine , lavender , tea tree , cannabis and lemongrass and is a component of many essential oils, e.g. B. Peru balsam oil and Cabreuva oil. It is used as a flavoring and fragrance .
biosynthesis
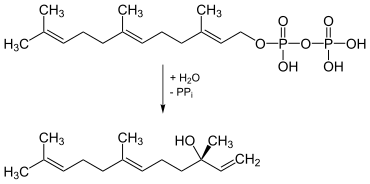
(3 S , 6 E ) -Nerolidol occurs naturally as an intermediate in the biosynthesis of 4,8-dimethyl-1,3 ( E ), 7-nonatriene. It is formed by the enzyme nerolidol synthase ( EC 4.2.3.48 ) from (2 E , 6 E ) - farnesyl pyrophosphate by splitting off pyrophosphate (PP i ).
Isomers
Nerolidol has a center of chirality and, if not specified in more detail, is present as a mixture of a total of four cis / trans and ( R ) / ( S ) isomers.
| Isomers of nerolidol | ||||||
| Surname | ( R ) - cis - Nerolidol | ( S ) - cis - Nerolidol | ( R ) - trans - Nerolidol | ( S ) - trans - Nerolidol | ||
| Structural formula | ||||||
| other names | (3 R , 6 Z ) - (-) - Nerolidol | (3 S , 6 Z ) - (+) - Nerolidol | (3 R , 6 E ) - (+) - Nerolidol | (3 S , 6 E ) - (-) - Nerolidol | ||
| CAS number | 132958-73-7 | 142-50-7 | 77551-75-8 | 1119-38-6 | ||
| 3790-78-1 [( Z ) -isomers] | 40716-66-3 [( E ) -isomers] | |||||
| 7212-44-4 (mixture of isomers) | ||||||
| PubChem | 12227246 | 5356544 | 11241545 | 5281525 | ||
| 8888 (mixture of isomers) | ||||||
| odor | intensely flowery, sweet and fresh | pleasant, woody, warm and musty | woody, green and bark | slightly sweet, mild and flowery | ||
|
GHS labeling |
|
|
||||
| H and P phrases | no H-phrases | 315-319-335 | ||||
| no P-phrases | 261-305 + 351 + 338 | |||||
Individual evidence
- ↑ a b c Entry on nerolidol. In: Römpp Online . Georg Thieme Verlag, accessed on June 22, 2014.
- ↑ a b data sheet cis-nerolidol (PDF) from Carl Roth , accessed on February 18, 2018.
- ↑ Entry on nerolidol, mixture of isomers in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ^ Entry on nerolidol in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ The European Bioinformatics Institute: EC 4.2.3.48, (3S, 6E) -nerolidol synthase .
- ↑ Wolfgang Legrum: Fragrances, between stink and fragrance , Vieweg + Teubner Verlag (2011) pp. 34–35, ISBN 978-3-8348-1245-2 .
- ^ Entry on trans-Nerolidol in the GESTIS substance database of the IFA , accessed on February 8, 2018(JavaScript required) .