Isobornyl acetate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Isobornyl acetate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 12 H 20 O 2 | |||||||||||||||
| Brief description |
colorless liquid with a characteristic odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 196.29 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.98 g cm −3 |
|||||||||||||||
| boiling point |
215 ° C |
|||||||||||||||
| Vapor pressure |
0.13 mbar (20 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.4635 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
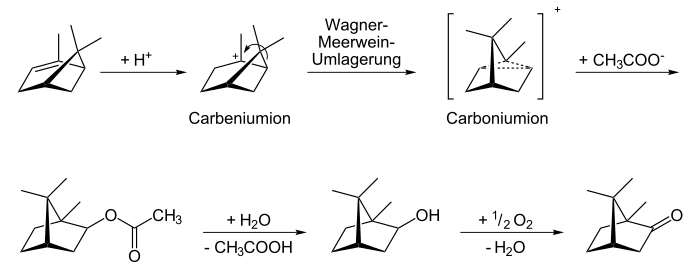
Isobornyl acetate is a chemical compound from the group of carboxylic acid esters and is isomeric to boronyl acetate . It is the ethyl ester of the terpene isoborneol .
Isomers
Isobornyl acetate is always exo -configured, the endo -configuration is called bornyl acetate or boronyl acetate . There are two isomeric forms, (-) - and (+) - isobornyl acetate [synonyms: (1 S , 2 S , 4 S ) - or (1 R , 2 R , 4 R ) -boronyl acetate], which are often referred to as 1 : 1 mixture ( racemate ) occur.
Extraction and presentation
Isobornyl acetate is made from camphene , producing more than 1000 tons per year.
properties
Isobornyl acetate is a colorless to yellowish liquid with a characteristic odor, which is very poorly soluble in water. It has a dynamic viscosity of 8.5 mPa · s at 20 ° C.
use
Isobornyl acetate is contained in odoriferous compositions (bath preparations, soap perfumes, sprays) and is used as an intermediate in the production of camphor .
safety instructions
The vapors of isobornyl acetate can form an explosive mixture with air ( flash point 88 ° C, ignition temperature 430 ° C).
Individual evidence
- ↑ a b c d e f g h i j k Entry on isobornyl acetate in the GESTIS substance database of the IFA , accessed on February 8, 2017(JavaScript required) .
- ↑ a b Toxicological evaluation of isobornyl acetate (PDF) at the professional association raw materials and chemical industry (BG RCI), accessed on August 26, 2010.
- ↑ Isobornyl acetate data sheet from Sigma-Aldrich , accessed on August 26, 2010 ( PDF ).
- ↑ Isobornyl acetate data sheet (PDF) from Merck , accessed on April 6, 2011.
