Chamazulen
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
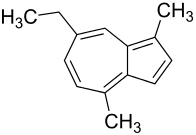
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Chamazulen | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 14 H 16 | |||||||||||||||
| Brief description |
brown-black needles |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 184.3 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
0.9883 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
127-129 ° C |
|||||||||||||||
| boiling point |
161 ° C (16 mbar) |
|||||||||||||||
| solubility | ||||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Chamazulene is a brown-black and in solution blue-violet component of the essential oil of chamomile flowers with anti-inflammatory effects, which belongs to the group of polycyclic aromatic hydrocarbons ( PAHs ) and terpene derivatives . The IUPAC name is 7-ethyl-1,4-dimethylazulene. The trivial name Chamazulen is a composite word from Cham omilla , the Latin names of the genuine chamomile , the French word azu r for sky blue , and the notes len (from Alk s ).
history
In 1863 the English chemist Septimus Piesse coined the name azulene for the blue substance from chamomile oil . However, the structure of chamazulene was only confirmed in 1953 as 7-ethyl-1,4-dimethylazulene.

It was found out relatively early on that the blue color only develops during extraction and distillation and that the azulene is therefore not genuinely present in the chamomile.
Extraction and presentation
The preliminary stage of chamazulen is contained as matricin in chamomile flowers and yarrow , among other things . The actual product is obtained from this during the distillation:
properties
Chamazulene forms brown-black needles that decompose quickly when exposed to air, light and temperature. It has anti-inflammatory ( antiphlogistic ) effect and is therefore next to Bisabolol and matricin one of the main active ingredients in camomile blossom oil.
Use and safety information
Chamomile flower oil is arguably one of the most frequently used and oldest natural medicines. The effect is based on a mixture of different substances (mostly flavonoids and terpenes or terpene derivatives); the anti-inflammatory effect is mainly due to the substances bisabolol, matricin and chamazulene it contains. Bisabolol and Matricin have the highest activity; Compared to matricin, chamazulen only has about 50% of its anti-inflammatory effects. In animal experiments, high doses of chamazulene caused breathing disorders in rabbits and mice.
Web links
Individual evidence
-
^ A b
J. Romo, AR de Vivar, PJ Nathan: The constituents of Zaluzania augusta . In: Tetrahedron . tape 23 , no. 1 , January 1967, p. 29-36 , doi : 10.1016 / s0040-4020 (01) 83283-x (English).
Quote: Crystallization of VIII from MeOH afforded dark brown needles (15 mg), mp 127-129 °. & chamazulene (VIII)
- ↑ a b S. Kalsi, Sunila Sharma, Gurdeep Kaur: Isodehydrocostus lactone and isozaluzanin C, two guaianolides from Saussurea lappa . In: Phytochemistry . tape 22 , no. 9 , 1983, pp. 1993–1995 , doi : 10.1016 / 0031-9422 (83) 80031-4 (English).
- ↑ a b c Lutz Roth (Hrsg.), Gabriele Rupp (Hrsg.): Roth collection of natural products data: concise descriptions and spectra. VCH, Weinheim 1995, ISBN 3-527-28180-0 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b c Rastitel'nye Resursy. Plant Resources. Vol. 3, p. 67, 1967.
- ↑ a b c Entry on Chamazulen in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ^ Septimus Piesse : On the Coloring Principle of Volatile Oils . In: Chemical News . No. 8 , 1863, p. 245 ( online [accessed March 2, 2015]).
- ^ Albert Gossauer: Structure and reactivity of biomolecules , Verlag Helvetica Chimica Acta, Zurich, 2006, p. 166, ISBN 978-3-906390-29-1 .
- ↑ a b V. Boltshauser: Wound healing with chamomile (PDF; 149 kB), on sprechzimmer.ch.
