Carvacrol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
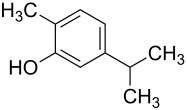
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Carvacrol | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 10 H 14 O | |||||||||||||||
| Brief description |
after thyme smelling liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 150.22 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.9772 g cm −3 (20 ° C ) |
|||||||||||||||
| Melting point |
~ 0 ° C |
|||||||||||||||
| boiling point |
237-238 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.5230 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
The carvacrol is a terpenoid natural products , known under different names. The systematic name ( IUPAC ) of carvacrol is 2-methyl-5- (1-methylethyl) phenol. The compound represents a structural isomer ( constitutional isomer ) to thymol .
Occurrence
Carvacrol is found in thyme ( Thymus ), winter savory ( Satureja montana ), summer savory ( Satureja hortensis ), oregano ( Origanum ), common catnip ( Nepeta cataria ) and goose feet (e.g. Chenopodium ambrosioides ). Oils obtained from these plant species can contain up to 85% carvacrol. A plant with a particularly high carvacrol content is the Cretan mountain tea Malotira ( Sideritis syriaca ).
Properties and structure
Carvacrol is a higher homologue of o -cresol . Legal regulations regarding carvacrol generally assume that thymol is harmless.
With iron (III) chloride, Carvacrol gives an olive green color.
Biological importance
Carvacrol has many uses, mainly as a biocide . It shows effects as an antifungal , insecticide , antibiotic and as an anthelmintic . In addition, Carvacrol inhibits the enzyme cyclooxygenase-2, which mediates inflammation reactions, and therefore corresponds in its effect to the anti-inflammatory drugs celecoxib and etoricoxib , which u. a. can be used against rheumatism and gout .
Individual evidence
- ↑ entry to carvacrol in CosIng database of the European Commission, accessed on 21 March 2020th
- ↑ a b c Entry on Carvacrol. In: Römpp Online . Georg Thieme Verlag, accessed on June 19, 2014.
- ↑ a b David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-316.
- ↑ a b The Merck Index Twelfth Edition. Merck Research Laboratories, 1996, ISBN 0-911910-12-3 , entry 1923.
- ↑ a b Carvacrol data sheet at Sigma-Aldrich , accessed on June 7, 2017 ( PDF ).
- ↑ a b c Entry on carvacrol in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ^ PM Jenner, EC Hagan, Jean M. Taylor, EL Cook, OG Fitzhugh: Food flavors and compounds of related structure I. Acute oral toxicity. In: Food and Cosmetics Toxicology . Vol. 2, 1964, p. 327.
- ^ GSB Viana, FF Matos, WL Araujo, FJA Matos, AA Craveiro: Essential Oil of Lippia grata: Pharmacological Effects and Main Constituents. In: Quarterly Journal of Crude Drug Research . Vol. 19, 1981, p. 1.
- ↑ Roger James, John B. Glen: Synthesis, biological evaluation, and preliminary structure-activity considerations of a series of alkylphenols as intravenous anesthetic agents. In: Journal of Medicinal Chemistry . Vol. 23, 1980, p. 1350.
- ↑ MS Owolabi, L. Lajide, MO Oladimeij, WN Setzer, MC Palazzo, RA Olowu, A. Ogundajo: Volatile constituents and antibacterial screening of the essential oil of Chenopodium ambrosioides L. growing in Nigeria. In: Nat. Prod. Commun. tape 4 , no. 7 , 2009, p. 989-992 , PMID 19731609 .
- ↑ H. Beyer, W. Walter: Textbook of organic chemistry. S. Hirzel Verlag, Stuttgart 1981, ISBN 3-7776-0356-2 , p. 643.
- ↑ thymol; Exemption from the Requirement of a Tolerance. In: Federal Register . 71 (11), 2006, pp. 2889-2895.
- ↑ Harry Auterhoff , Joachim Knabe, Hans-Dieter Höltje: Textbook of pharmaceutical chemistry. Wissenschaftliche Verlagsgesellschaft, Stuttgart 1999, ISBN 3-8047-1645-8 .
- ↑ S. Kordali, A. Cakir, H. Ozer, R. Cakmakci, M. Kesdek, E. Mete: Antifungal, phytotoxic and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p- cymene. In: J. Bior. Tech. tape 99 , no. 18 , 2008, p. 8788-8795 , doi : 10.1016 / j.biortech.2008.04.048 , PMID 18513954 .
- ↑ MM Obaidat, JF Frank: Inactivation of Escherichia coli O157: H7 on the Intact and Damaged Portions of Lettuce and Spinach Leaves by Using Allyl Isothiocyanate, Carvacrol, and Cinnamaldehyde in Vapor Phase. In: J. Food Prot. Band 72 , no. 10 , 2009, p. 2046-2055 , PMID 19833026 .
- ↑ M. Hotta, R. Nakata, M. Katsukawa, K. Hori, S. Takahashi, HJ Inoue: Carvacrol, a component of thyme oil, activates PPARalpha and gamma and suppresses COX-2 expression. In: Lipid Res. 51 (1), Jan 2010, pp. 132-139. PMID 19578162 .


