Linalyl acetate
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
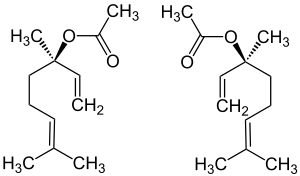
|
||||||||||
| ( R ) shape (left) and ( S ) shape (right) | ||||||||||
| General | ||||||||||
| Surname | Linalyl acetate | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 12 H 20 O 2 | |||||||||
| Brief description |
colorless liquid with a pleasant smell of bergamot |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 196.29 g mol −1 | |||||||||
| Physical state |
liquid |
|||||||||
| density |
0.9 g cm −3 |
|||||||||
| Melting point |
−100 ° C |
|||||||||
| boiling point |
220 ° C |
|||||||||
| Vapor pressure |
1 mbar (20 ° C) |
|||||||||
| solubility |
|
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| Toxicological data | ||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Linalyl acetate a Mono terpenoid - ester having the empirical formula C 12 H 20 O 2 . It is a colorless liquid with a fresh, sweet odor.
Occurrence
Linalylacetat found as a major component in lavender oil (30 - 60%), the lavender oil (25 - 50%), the clary sage (up to 75%) and bergamot (30 - 45%) and comes in addition to many other essential oils before .
Extraction and presentation
Linalyl acetate can be obtained from the essential oils mentioned above.
Synthetically is Linalylacetat can by esterification of linalool win, needs to be taking care of mild reaction conditions, as linalool unsaturated tertiary alcohol to dehydration and cyclization tendency. Suitable synthesis routes starting from linalool would be, for example, the reaction with ethenone , with boiling acetic anhydride while distilling off the acetic acid formed, or a transesterification using tert-butyl acetate in the presence of sodium methoxide .
It can be obtained from dehydrolinalool by esterification with acetic anhydride and subsequent partial hydrogenation .
The reaction of β-pinene with acetic acid and sodium acetate in the presence of copper (I) chloride is also used , linalyl acetate being formed with a yield of 75-80%.
Linalyl acetate is one of the chemical substances that are produced in large quantities (“ High Production Volume Chemical ”, HPVC) and for which the Organization for Economic Cooperation and Development (OECD) collects data on possible hazards (“ Screening Information Dataset ”, SIDS ) was made.
use
Linalyl acetate is used extensively in perfumery . Due to its good stability in an alkaline environment, it is also often used in soaps or detergents .
safety instructions
The vapors of linalyl acetate can form an explosive mixture with air ( flash point 94 ° C, ignition temperature 225 ° C).
Individual evidence
- ↑ Entry on LINALYL ACETATE in the CosIng database of the EU Commission, accessed on February 26, 2020.
- ↑ a b entry on linalyl acetate. In: Römpp Online . Georg Thieme Verlag, accessed on November 3, 2014.
- ↑ a b c d e f g h i Entry on linalyl acetate in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ^ A b Richard J. Lewis, Sr .: Hawley's Condensed Chemical Dictionary . 15th edition. Wiley-Interscience, 2007, ISBN 978-0-471-76865-4 (English).
- ^ A b Karl-Georg Fahlbusch, Franz-Josef Hammerschmidt, Johannes Panten, Wilhelm Pickenhagen, Dietmar Schatkowski, Kurt Bauer, Dorothea Garbe and Horst Surburg: Flavors and Fragrances . In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley-VCH Verlag GmbH & Co. KGaA, 2003, ISBN 3-527-30673-0 , doi : 10.1002 / 14356007.a11_141 .
- ↑ patent GB878680 : Production of linalyl acetate. Published October 4, 1961 , Applicant: Distillers Company, Inventor: Peter Nayler.
- ↑ Patent DE2025727 : Process for esterification of tertiary terpenic alcohols. Published December 3, 1970 , Applicant: Rhône-Poulenc Ind., SA, Inventor: PS Gradeff, B. Finer.
- ↑ Patent DE1768980 : Process for the production of carboxylic acid esters of olefinically unsaturated tertiary alcohols. Published on August 12, 1971 , Applicant: BASF, Inventor: H. Pasedach.
- ↑ patent GB774621 : Novel Acetylenic ester and the conversion thereof into Further ester. Published on May 15, 1957 , Applicant: Hoffmann-La Roche, Inventor: JA Birbiglia, GO Chase, J. Galender.
- ↑ Patent US3076839 : Process for producing allylic esters. Published February 5, 1963 , Applicant: Glidden Co, Inventor: RL Webb.
- ↑ OECD : Screening Information Dataset (SIDS) Initial Assessment Report (SIAR) for Linalyl Acetate , accessed on October 3, 2014.

