Caran
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
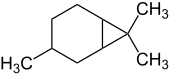
|
||||||||||
| Structure without showing the stereochemical configuration | ||||||||||
| General | ||||||||||
| Surname | Caran | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 10 H 18 | |||||||||
| Brief description |
colorless, pleasant smelling liquid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 138.25 g mol −1 | |||||||||
| Physical state |
liquid |
|||||||||
| density |
0.841 g cm −3 |
|||||||||
| boiling point |
169 ° C |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Caran is a bicyclic, bridged hydrocarbon that forms the backbone for a group of terpenes . With the empirical formula C 10 H 18 , it is a monoterpene , i.e. a constitutional isomer of Bornane and Pinane .
structure
Carane and the terpenes derived from it can also be understood as derivatives of the hydrocarbon bicyclo [4.1.0] heptane. This parent compound consists of a six-membered ring (cyclohexane ring) and a three-membered ring (cyclopropane ring), which are cis -linked ( fused ). The carbon atoms of the six-membered ring are not in one plane. In the caran there are still two geminal methyl groups on carbon atom 7 and one methyl group on C-3. If the carbon atoms of the six-membered ring are projected into a plane, the C-3-methyl (= C-10) can be on the same side as C-7 (of the three-membered ring), or on the opposite side. Therefore, two isomers ( diastereomers ) of the bicyclic hydrocarbon are possible, which were referred to as cis -caran and trans -caran, respectively. Both isomers are chiral.
| Isomers of caran | ||
| Surname | cis caran | trans -Caran |
| other names |
|
|
| Structural formula |  |

|
| CAS number | 18968-24-6 2778-68-9 (-) - cis |
18968-23-5 (+) - trans |
| 554-59-6 (unspec.) | ||
| PubChem | 12302437 | 12302439 |
| 79043 (unspec.) | ||
| Refractive index | 1.4550 at 20 ° C | 1.4565 at 20 ° C |
| Optical activity [α] D 20 | −37.7 ° | + 63.5 ° |
Occurrence and manufacture
The hydrocarbons do not seem to occur in nature, but the unsaturated hydrocarbon 3-carene as a component of turpentine oils, as well as some alcohols and ketones .
A synthesis of cis -caran starts from 3-carene, which after hydroboration and oxidation gave the alcohol cis -caran- trans -4-ol. This was deoxygenated by reducing its tosylate with lithium aluminum hydride.
trans -caran was obtained from caran-2-one by Wolff-Kishner reduction . However, the ketone is not a natural substance , but was produced synthetically.
The simplest method to produce these bicyclic hydrocarbons should be the hydrogenation of 3-carene. However, it is not selective. A previous isomerization (equilibration) to 2-carene was observed in the case of the heterogeneous catalytic hydrogenation. In the end, cis -carane is formed in addition to 1,1,4-trimethylcycloheptane, i.e. H. the C-1 / C-6 bond is partially split hydrogenolytically. In heterogeneous catalytic hydrogenation with platinum black , for example, a mixture of cis -caran (65%), trans -caran (32.5%) and 1,1,4-trimethylcycloheptane (2.5%) is to be formed. Pure cis and trans caran can be obtained by fractional distillation of the mixture .
literature
- D. Whittaker: The Monoterpenes in: AA Newman (Ed.): Chemistry of Terpenes and Terpenoids . P. 35, Academic Press, London 1972, ISBN 0-12-517950-2
Individual evidence
- ↑ a b c Entry on Caran. In: Römpp Online . Georg Thieme Verlag, accessed on October 31, 2012.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ John Lionel Simonsen: The Terpenes Volume II The Dicyclic Terpenes, Sesquiterpenes and their ... University Press, 1947, pp. 54 ( limited preview in Google Book search).
- ^ A b c d e f W. Cocker, PVR Shannon and PA Staniland: The chemistry of terpenes. Part II. The physical properties of some cis - trans substituted cyclohexanes . J. Chem. Soc. C, 1966, 946-949, doi : 10.1039 / J39660000946 .
- ↑ Wesley Cocker, PVR Shannon, PA Staniland: The chemistry of terpenes. Part I. Hydrogenation of the pinenes and the carenes , J. Chem. Soc. C, 1966, 41-47, doi : 10.1039 / J39660000041 .
- ↑ II Bardychev, EF Buinova, AL Pertsovskii: Synthesis and study of the properties of cis and trans caranes , Vesti Acad. Navuk Belarus. SSR Ser. Khim. Navuk, 1970, 99-101. Quoted from Chemical Abstracts , Vol 74 (1971), Paper No. 112223j ( 74 : 112223j).
