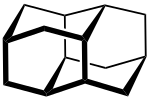
Diamantan
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| General | ||||||||||
| Surname | Diamantan | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 14 H 20 | |||||||||
| Brief description |
colorless crystals |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 188.31 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| density |
1.21 g cm −3 (under normal conditions) |
|||||||||
| Melting point |
244 ° C |
|||||||||
| boiling point |
272 ° C |
|||||||||
| solubility |
|
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Diamantan (also Congressan ) is a hydrocarbon compound of organic chemistry , which is assigned to the group of diamondoids . Like all diamondoids, it has a cage-like structure that can be found in the crystal lattice of a diamond . Due to this special structure, the colorless solid is a research subject in chemistry.
history
Adamantane was discovered in 1933 as the simplest diamondoid by the Slovak chemist Stanislav Landa and fascinated some chemists because of its special cage-like structure. This also included the Swiss chemist Vladimir Prelog , who first succeeded in synthesizing adamantane in 1944. He made the structure of a C14 body, which can be found in the diamond crystal lattice and contains two adamantane units, the emblem of the 19th IUPAC Congress in 1963. Since this compound was unknown at the time, it was initially called Congressan. The conference called for a synthesis of the Congressans.
Chris Cupas, Paul von Ragué Schleyer and David J. Trecker synthesized Congressan for the first time in 1965 with a yield of 1% - by isomerizing a mixture of norbornene - [2 + 2] dimers with the catalytic effect of aluminum chloride . A year later, another organic compound with three adamantane building blocks was found, whereupon these adamantolgenic compounds were assigned to the new group of diamondoids. A simple, general nomenclature was developed for the representatives of this group:
- Adamantane
- Congressan (with two adamantane components) was renamed Diamantan.
- The new compound with three adamantane building blocks was called triamantane.
- etc.
In 1966, Diamantan was first isolated from petroleum . In the same year a new synthesis variant was developed through which Diamantane can be formed with a yield of 10%. In 1970 it was finally possible to synthesize Diamantan in sufficient quantities so that from this point on the substance could be researched more easily.
Occurrence
Diamantan occurs mainly in unprocessed petroleum and in gas condensates. It is believed that the substance is created in the natural process of catagenesis .
Extraction and presentation
Due to its particularly high thermal stability, Diamantan can be isolated from crude oil to natural gas during hydrocracking . According to a method by Paul von Ragué Schleyer, diamondane can be produced by the rearrangement of various pentacyclic tetradecanes under the catalytic action of Lewis acids . It has been found that the diamantane synthesis via hydrogenated binary S ( 3a , 3b ) is most suitable. Approx. 70% Diamantan is obtained here.
The synthesis begins with the preparation of Binor-S (heptacyclo [8.4.0.0 2.12 .0 3.7 .0 4.6 .0 5.9 .0 11.13 ] tetradecane, 2 ) by dimerization of norbornadiene ( 1 ) with the catalytic effect of cobalt bromide - triphenylphosphine and boron trifluoride diethyl etherate . The next step is carried out hydrogenation (for tetrahydro-Binor-S-isomers 3a - 3d ) using a solution of glacial acetic acid and hydrochloric acid with a platinum (IV) oxide - catalyst in a hydrogen environment. For steric reasons, the isomers 3a and 3b are mainly formed . Under the catalytic effect of aluminum bromide in cyclohexane or carbon disulfide , a rearrangement occurs, in which the main product diamondane ( 4 ) is formed. The yield here is approx. 70%.
properties
As is known with diamondoids, their structures can be found as subunits in the diamond crystal lattice. This also applies to Diamantan:
This special structure results in a - compared to other hydrocarbons - high melting point of 244 ° C as well as considerable chemical and thermal stability.
| Standard enthalpy of formation | source |
|---|---|
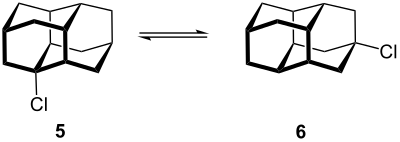
Derivatives of the inert diamondane can easily be prepared by e.g. B. a solution of the hydrogenated Binor-S ( 3a , 3b ) with aluminum chloride and dichloromethane is heated and after a few hours, acetyl chloride is added. The result is 1-chlorodiamantane ( 5 ) and 4-chlorodiamantane ( 6 ), which are produced in a 1: 1 ratio. The respective alcohols are formed by hydrolysis.
use
The area of application of Diamantan is diverse because it is chemically and thermally stable, retains its physical properties at high temperatures and dissolves well in organic solvents. Diamantan is used to improve the thermal stability of polymers and to produce thermosetting resins. In nanotechnology , diamondoids (e.g. adamantane and diamondane) are important molecular building blocks due to their small size and properties. Diamondoids also play a major role in the development of drugs and drug targeting . An example is 1,6-diamine diamond, which has anti-tumor activity and antibacterial activity.
Web links
Individual evidence
- ↑ Entry on Diamantane at TCI Europe, accessed on June 5, 2017.
- ↑ a b G. A. Mansoori: Diamondoid Molecules. In: Advances in Chemical Physics . Volume 136, 2007, pp. 207-258; (PDF)
- ↑ PR Schreiner, LV Chernish, PA Gunchenko, EY Tikhonchuk, H. Hausmann, M. Serafin, S. Schlecht, JEP Dahl, RMK Carlson, AA Fokin: Overcoming lability of extremely long alkane carbon-carbon bonds dispersion through forces. In: Nature . Volume 477, No. 7364, 2011, pp. 308-311, doi: 10.1038 / nature10367 .
- ^ WS Wingert: Gc-ms analysis of hydrocarbons in Smackover petroleums. In: Fuel , Vol. 71, No. 1, 1992, pp. 37-43, doi: 10.1016 / 0016-2361 (92) 90190-Y .
- ↑ YC Chan, KKH Choy, AHC Chan, KM Ng, S. Liu, SF Sciamanna, JE Dahl, RMK Carlson: Solubility of Diamantane, Triamantane, Tetramantane, and Their Derivatives in Organic Solvents. In: Journal of Chemical & Engineering Data . Volume 53, No. 8, 2008, pp. 1767-1771, doi: 10.1021 / je800277a .
- ↑ P. Karasek, J. Planeta, M. Roth: Solubilities of Adamantane and Diamantane in Pressurized Hot Water. In: Journal of Chemical & Engineering Data . Volume 53, No. 3, pp. 816-819, doi: 10.1021 / je700709m .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ V. Prelog, R. Seiwerth: The synthesis of Adamantas. In: Reports of the German Chemical Society . Volume 74, No. 10, 1941, pp. 1644-1648, doi: 10.1002 / cber.19410741004 .
- ^ V. Prelog: Conformation and reactivity of medium-sized ring compounds. In: Pure and Applied Chemistry . Volume 6, No. 4, 1963, pp. 545-560, doi: 10.1351 / pac196306040545 .
- ↑ a b c d T. M. Gund, E. Osawa, W. Van Zandt Jr., P. v. R. Schleyer: Diamantane. 1. Preparation of Diamantane. Physical and Spectral Properties. In: Journal of Organic Chemistry . Volume 39, No. 20, 1974, pp. 2979-2987, doi: 10.1021 / jo00934a009 .
- ↑ C. Cupas, P. v. R. Schleyer, DJ Trecker: Congressane. In: Journal of the American Chemical Society . Volume 87, No. 4, 1965, pp. 917-918, doi: 10.1021 / ja01082a042 .
- ^ BC Anderson, O. Vogl, DM Simons: Synthesis of Hexaoxadiamantanes. In: Tetrahedron Letters . Volume 7, No. 4, 1966, pp. 415-418, doi: 10.1016 / S0040-4039 (00) 72956-X .
- ↑ S. Hála, S. Landa, V. Hanuš: Isolation of tetracyclo [6.3.1.0 2.6 .0 5.10 ] dodecane and pentacyclo [7.3.1.1 4.12 .0 2.7 .0 6.11 ] tetradecane (Diamantane) from petroleum. In: Angewandte Chemie , Volume 78, No. 23, 1966, pp. 1060-1061, doi: 10.1002 / anie.19660782309 .
- ↑ a b T. M. Gund, E. Osawa, W. Van Zandt Jr., P. v. R. Schleyer: A Convenient, High-Yield Preparation of Diamantane (Congressane). In: Tetrahedron Letters . 1970, Vol. 11, No. 44, pp. 3877-3880, doi: 10.1016 / S0040-4039 (01) 98613-7 .
- ↑ a b J. E. Dahl, JM Moldowan, KE Peters, GE Claypool, MA Rooney, ME Michael, MR Mello, ML Kohnen: Diamondoid hydrocarbons as indicators of natural oil cracking. In: Nature . Volume 399, 1999, pp. 54-57, doi: 10.1038 / 19953 .
- ↑ AIA Petrov, OA Arefjey, ZV Yakubson: Hydrocarbons of adamantanes Series as indices of Petroleum Catagensis Process. In: Advances in Organic Geochemistry 1973 - Actes du 6e Congrès International de Geochimie Organique 18-21 September 1973 Rueil-Malmaison. Éditions Technips, Paris 1974, ISBN 2-7108-0258-9 , pp. 517-522.
- ↑ TM Gund, W. Thielecke, P. v. R. Schleyer: Diamantane: PENTACYCLO [7.3.1.1 4.12 .0 2.7 .0 6.11 ] TETRADECANE [Butanetetraylnaphthalene, 3,5,1,7- [1,2,3,4] -decahydro-] . In: Organic Synthesis , Volume 53, 1973, p. 30, doi: 10.15227 / orgsyn.053.0030 .
- ^ A b T. Clark, TM Knox, MA McKervey, H. Mackle, JJ Rooney: Thermochemistry of bridged-ring substances. Enthalpies of formation of some diamondoid hydrocarbons and of perhydroquinacene. Comparisons with data from empirical force field calculations. In: Journal of the American Chemical Society . Volume 101, No. 9, 1979, pp. 2404-2410, doi: 10.1021 / ja00503a028 .
- ^ T. Courtney, DE Johnston, MA McKervey, JJ Rooney: The Chemistry of Diamantane. Part 1. Synthesis and Some Functionalization Reactions. In: Journal of the Chemical Society . 1972, pp. 2691-2696, doi: 10.1039 / P19720002691 .