Diamondoids
The diamondoids ( Diamond-like , engl. Diamondoids ) are a group of substances of mineral cycloalkanes in which the carbon skeleton is equal of a diamond, as well as their substituent . The simplest diamondoid is adamantane . Some diamondoids occur in small quantities in petroleum and rock crystals . The diamondoids are to be used in nanotechnology , they are already in use for the production of pharmaceuticals . The diamondoids can be understood as diamond splinters, on the outer surface of which hydrogen has accumulated. There are also mineral, diamond-like cycloalkenes , the simplest representative of which is the adamant .
The homologues of adamantane form a homologous series with the general formula C 4n + 6 H 4n + 12 = C m H m + 6 (n = number of adamantane formula units; m = number of carbon atoms in the molecule). According to its structure, the formula for adamantane can also be written like this: (CH) 4 (CH 2 ) 6 ; this reveals the relationship between its structure and that of hexamethylenetetramine : N 4 (CH 2 ) 6 (this can also be referred to as tetraazaadamantane).
Some diamondoids:
- n = 1 → adamantane (C 10 H 16 )
- n = 1.5 → Icean (C 12 H 18 )
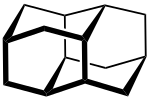
- n = 2 → BC-8 (C 14 H 20 )
- n = 2 → Diamantan (C 14 H 20 ) (also Diadamantan )
- n = 3 → triamantane (C 18 H 24 ) (also triadamantane )
- n = 4 → isotetramantane (C 22 H 28 )
- n = 5 → pentamantane (C 26 H 32 )
- n = 6 → super adamantane (C 30 H 36 )
From five diamond cages (n = 5), the empirical formula for diamondoids loses its general validity, as individual structures exist which, due to the special arrangement of the adamantane units, require fewer atoms than the formula says. An example of this is cyclohexamantane (C 26 H 30 ), the six diamond cages or adamantane units of which are arranged in a quasi-circle, which saves building material. From pentamantane onwards, the empirical formula given above is still correct for many structures, but is generally to be understood as an upper limit.
Alan Marchand was able to prepare cis - and trans -1,2-di (1-adamantyl) ethene, which is interesting because of its steric repulsion.
Web links
- Cluster and Nanocrystal Research Group, Technical University Berlin
- Graduate Molecular Modeling: Adamantane ( Memento from February 25, 2011 in the Internet Archive )