Trinorbornane
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|

|
|||||||
| General | |||||||
| Surname | Trinorbornane | ||||||
| other names |
Tetracyclo [5.2.2.0 1.6 .0 4.9 ] undecane ( IUPAC ) |
||||||
| Molecular formula | C 11 H 16 | ||||||
| Brief description |
colorless, crystalline solid |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 148.24 g mol −1 | ||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Trinorbornane is a tetracylic , saturated hydrocarbon made up of cyclopentane and cyclohexane rings. The name of the compound is derived from the three norbornane structural elements that are present in the molecule. The name trinorbornane should not be confused with the alternative name for norbornane, 8,9,10-trinorbornane.
synthesis
The synthesis of trinorbornane in a multi-step reaction sequence was published in 2017.
First, dimethyl malonate 1 under basic conditions in DMF at room temperature with ( E ) -4-chloro-3-methoxy-2-butenoate 2 implemented. The resulting ( E ) -3-methoxy-3-buten-1,1,4-tricarboxylic acid methyl ester 3 is cyclized in a solution of sodium methoxide in methanol at 60 ° C. to give 4-methoxy-2-oxo-cyclopent-3-ene carboxylic acid methyl ester 4 .
By deprotonation of the cyclopentenone derivative 4 with potassium hydride in DMF using 4-bromo-1-butene 5 , the alkylation of 1- (but-3-enyl) -4-methoxy-2-oxo-cyclopent-3-enecarboxylic acid methyl ester 6 . The saponification of the ester group followed by a decarboxylation is carried out by reaction with potassium hydroxide in ethanol under reflux. At the same time, the methoxy group is converted into an ethoxy group and 5- (but-3-enyl) -3-ethoxycyclopent-2-enone 7 is obtained . The compound 7 is reacted with lithium diisopropylamide deprotonated and (LDA) at -78 ° C at 0-25 ° C in the presence of 2-imidazolidinone, 1,3-dimethyl (DMI) with phenyl vinyl sulphone 8 in the sense of a Michael addition for connection 9 reacted .
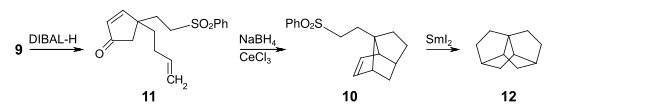
With diisobutylaluminum hydride (DIBAL-H) in toluene , the selective reduction to 4- (but-3-en1-yl) -4- (2- (phenylsulfonyl) ethyl) cyclopent-2-enone 10 is possible . Under the conditions of the Luche reduction , cerium (III) chloride and sodium borohydride are used to reduce the cyclopentenone structural element of 10 to the corresponding cyclopenten-2-ol. Without intermediate isolation, this is dehydrated, the resulting cyclopentadiene derivative cyclizing with the 3-butene side chain in the sense of an intramolecular Diels-Alder reaction to give compound 11 . In the last step, the phenyl sulfone group is reduced with samarium (II) iodide to form a 2-ethyl radical unit. This cyclizes to the target compound 12 .
The isolated yield over all stages is 7%.
properties
Trinorbornane is an example of a compound that exhibits axial chirality . The molecule has a C 2 symmetry .
The carbon atom in the 1-position is situated on the chirality of the molecule, one that of a 1 -S a and a 1- R a - enantiomer can speak. In addition, there are four other centers of chirality in the molecule (C-4,6,7,9), which, however, have the identical configuration in both enantiomers. The IUPAC name of the two trinorbornane enantiomers is (1 S a , 4 S , 6 R , 7 S , 9 R ) -Tetracyclo- [5.2.2.0 1,6 .0 4,9 ] undecane and (1 R a , 4 S , 6 R , 7 S , 9 R ) -Tetracyclo- [5.2.2.0 1.6 .0 4.9 ] undecane. A racemate is obtained in the synthesis of the compound .
Individual evidence
- ↑ a b c d Lorenzo Delarue Bizzini, Thomas Müntener, Daniel Häussinger, Markus Neuburger, Marcel Mayor: Synthesis of trinorbornane . In: Chem. Commun. tape 53 , no. 83 , 2017, p. 11399 , doi : 10.1039 / c7cc06273g ( unibas.ch [PDF]).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ Modification of the Parent Structure. Rule F-4.2. In: IUPAC Nomenclature of Organic Chemistry. Advanced Chemistry Development, accessed December 4, 2019 .