Diisobutyl aluminum hydride
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
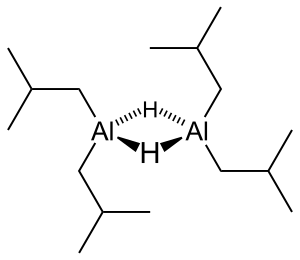
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Diisobutyl aluminum hydride | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 8 H 19 Al | ||||||||||||||||||
| Brief description |
Colorless liquid which can spontaneously ignite in air |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 142.22 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.79 g cm −3 |
||||||||||||||||||
| Melting point |
−80 ° C |
||||||||||||||||||
| boiling point |
114 ° C (at 1 h Pa ) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Diisobutylaluminum hydride (abbr.DIBAL, DIBAL-H, DIBALH, DIBAH) is a reducing agent with the chemical formula ( i -Bu) 2 AlH ( i -Bu = isobutyl). DIBAL is mainly used in organic synthesis for various reductions (see: Hydroalumination ) and in terms of reactivity it lies between lithium aluminum hydride (LiAlH 4 ) and sodium borohydride (NaBH 4 ).
A typical field of application of DIBAL is the direct reduction of esters to aldehydes . In order to prevent a consecutive reduction of the aldehydes formed to alcohols , the reaction is carried out at −78 ° C. When using LiAlH 4, for example, the esters are always further reduced to alcohols, while NaBH 4 has hardly any reactivity ( depending on the substituents) towards esters.
DIBAL is also frequently used to reduce nitriles to aldehydes. This allows the reaction to be controlled so that a DIBAL-H molecule only ever transfers one hydride ion. Because of the bulky isobutyl groups, sterically easily accessible, electrophilic centers are preferentially attacked.
DIBAL is an electrophilic reducing agent that reacts well with electron-rich compounds, while the reactivity with electron-poor substrates is rather low.
DIBAL is commercially available in substance, but more conveniently in solution (e.g. in toluene ). It can be synthesized from triisobutylaluminum by β-hydride elimination . DIBAL is sensitive to air and moisture and reacts violently with water.
Individual evidence
- ↑ a b c Entry on DIBAL. In: Römpp Online . Georg Thieme Verlag, accessed on May 2, 2014.
- ↑ a b c d Product Data Sheet Nouryon ( Memento from July 25, 2019 in the Internet Archive ).
- ^ A b c A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 .
- ↑ a b Data sheet Diisobutylaluminum hydride from Sigma-Aldrich , accessed on March 25, 2011 ( PDF ).

