Superphenals
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
|
|||||||
| General | |||||||
| Surname | Superphenals | ||||||
| Molecular formula | C 96 H 30 | ||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 1183.27 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
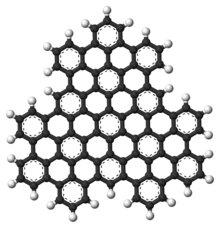
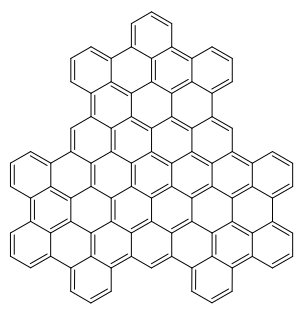
Superphenals is a very large representative of the polycyclic aromatic hydrocarbons (PAH). Formally, it can be seen as consisting of three fused superbenzenes (hexa-peri-hexabenzocoronene).
It can be viewed as an overlapping structure of three hexa-peri-hexabenzocoronen (HBC) symmetrically arranged around a center (also called superbenzene). These have also been known as the building blocks of molecular electronics since 2004, as they form self-assembling columns and nanotubes.

presentation
Klaus Müllen synthesized it as a basic building block for columns in liquid crystals , which like the molecule have a C 3 symmetry (threefold symmetry axis perpendicular to the flat molecule ). His first synthesis was carried out with “wet chemistry” and was described by him as quite simple using standard methods of the synthesis of benzoid PAHs (BPACs): Diels-Alder reaction of 1,2,3-triethinylbenzene with cyclopentadienone derivatives in the presence of o - xylenes . Precursors of the polybenzene compound are formed, which are then subjected to an oxidative cyclodehydrogenation under acidic conditions. Müllen generally pursued a program of cheaper production of building blocks for molecular electronics using methods of wet organic chemistry (instead of chemical vapor deposition ), preferably building blocks that have a tendency to self-assemble. It is an example of graphene nanostructures and was also generated by Müllen and co-workers using the soft landing mass spectrometry they developed on surfaces. Müllen and his group synthesize such graphene nanostructures (including conductive strips) in the context of organic electronics.
Occurrence
A natural occurrence is not known.
properties
Superphenals have a planar geometry. With 540,000 mesomeric boundary structures it has significantly more than superbenzene (250), supernaphthalene (16,100) and also than buckminster fullerene (12,500). The molecule has a threefold axis of symmetry (C 3 ) perpendicular to the molecule .
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b Milan Randić, Xiaofeng Guo: Giant benzenoid hydrocarbons. Superphenalene resonance energy . In: New Journal of Chemistry . tape 23 , no. 2 , 1999, p. 251-260 , doi : 10.1039 / A808949C .
- ↑ Shunji Ito, Peter Tobias Herwig, Thilo Böhme, Jürgen P. Rabe, Wolfgang Rettig, Klaus Müllen: Bishexa-peri-hexabenzocoronenyl: A “Superbiphenyl”. In: Journal of the American Chemical Society . 122, 2000, pp. 7698-7706, doi: 10.1021 / ja000850e .
- ↑ Jishan Wu, Mark D. Watson, Natalia Tchebotareva, Zhaohui Wang, Klaus Müllen: Oligomers of Hexa-peri-hexabenzocoronenes as “Super-oligophenylenes”: Synthesis, Electronic Properties, and Self-assembly. In: The Journal of Organic Chemistry . 69, 2004, pp. 8194-8204, doi: 10.1021 / jo0490301 .
- ↑ Jonathan P. Hill, Wusong Jin, Atsuko Kosaka, Takanori Fukushima, Hideki Ichihara, Takeshi Shimomura, Kohzo Ito, Tomihiro Hashizume, Noriyuki Ishii, Takuzo Aida: Self-Assembled Hexa-peri-hexabenzocoronene Graphitic Nanotube , Science , Volume 304, 2004 , Pp. 1481-1483, doi: 10.1126 / science.1097789 , PMID 15178796 .
- ^ Hans Joachim Wheels, Ali Rouhanipour, Anna Maria Talarico, Vincenzo Palermo, Paolo Samorì, Klaus Müllen: Processing of giant graphene molecules by soft-landing mass spectrometry. In: Nature Materials , Volume 5, 2006, pp. 276-280, doi: 10.1038 / nmat1597 ( figure ).
literature
- Milan Randić: Resonance energy of very large benzenoid hydrocarbons . In: International Journal of Quantum Chemistry . tape 17 , no. 3 , 1980, p. 549-586 , doi : 10.1002 / qua . 560170314 .
- Željko Tomović, Mark D. Watson, Klaus Müllen: Superphenalene-Based Columnar Liquid Crystals . In: Angewandte Chemie International Edition . tape 43 , no. 6 , 2004, p. 755-758 , doi : 10.1002 / anie.200352855 .
- German edition: Columnar liquid crystals based on superphenals, Angewandte Chemie, Volume 116, 2004, pp. 773-777