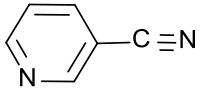
3-cyanopyridine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 3-cyanopyridine | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 4 N 2 | |||||||||||||||
| Brief description |
white to beige, crystalline solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 104.11 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density | ||||||||||||||||
| Melting point |
48-52 ° C |
|||||||||||||||
| boiling point |
|
|||||||||||||||
| Vapor pressure |
0.296 mm Hg (25 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.4460-1.4490 (20 ° C, 589 nm) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
3-cyanopyridine is an important precursor for nicotinaldehyde , nicotinic acid (niacin), and nicotinamide (niacinamide), as well as for plant protection products , such. B. the insecticide pymetrozine .
Occurrence and representation
Pyridine-3-sulfonic acid ( obtainable from pyridine and sulfur trioxide in the presence of mercury (II) sulfate at 235 ° C in yields of> 90%) reacts after neutralization with sodium hydroxide solution as the sodium salt when heated with mixtures of sodium cyanide and potassium cyanide in 45% strength Yield to 3-cyanopyridine.
The nucleophilic exchange of the bromine in 3-bromopyridine (by bromination with the ionic liquid N -octylquinolinium tribromide in 91% yield) by cyanide from copper (I) cyanide gives nicotinonitrile in 67% yield.
Substantially better yields (93%) achieved the reaction in the presence of organopalladium compound Pd 2 (dba) 3 [tris (dibenzylideneacetone) dipalladium (0)], the phosphine t-Bu 3 P (tri-tert. Butyl phosphine) and tributyltin chloride in acetonitrile at 80 ° C.
Acrylonitrile dimerizes in a head-to-tail arrangement e.g. B. in the presence of tricyclohexylphosphine PCy 3 in 77% yield to 2-methyleneglutaronitrile (2,4-dicyano-1-butene), which after chlorination to 2-chloro-2-chloromethylglutaronitrile with Lewis acids such. B. tin (IV) chloride or aluminum chloride reacts to form 3-cyanopyridine.
The oxidative ammonolysis of nicotine and its secondary alkaloids nornicotine , nicotyrin , anabasine , anatabine and myosmin from tobacco waste using vanadium (V) oxide / titanium dioxide catalysts with ammonia and hydrogen peroxide at 420 ° C generates nicotinonitrile in yields of up to 60%.
The specified synthetic routes are unsuitable for an industrial synthesis of 3-cyanopyridine because of expensive starting materials and reagents, low yields and high expenditure on equipment.
By reacting nicotinic acid with diphosphorus tetraiodide / ammonium carbonate , nicotinonitrile can be obtained in 88% yield, with copper (I) chloride and the silylating agent MSTFA (N-methyl-N- (trimethylsilyl) trifluoroacetamide) in 96% yield.
The elimination of water from nicotinic acid amide by means of phosphorus pentoxide produces 3-CP in 83 to 84% yield, with the sulfur trioxide - triethylamine complex yields of 95% are achieved.
The older variant of the 3-cyanopyridine synthesis from Lonza AG is based on this reaction.
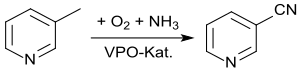
In addition to the dehydration of nicotinamide, the ammoxidation of 3-picoline in the vapor phase has established itself on an industrial scale . So-called VPO catalysts, based on vanadium (V) oxide and phosphorus pentoxide , which are doped with transition metal oxides, such as e.g. B. molybdenum , titanium , with the addition of zirconium or iron .
The ammoxidation of 3-methylpyridine is as high as possible in terms of catalyst composition, reaction time and reaction temperature (340-440 ° C), pressure (normal pressure) and the composition of the mixture of 3-picoline, ammonia, air or oxygen and water (up to 100 %), Selectivity (up to 100%) and yield (> 95%) optimized.
A modern synthetic route starts with 2-methylpentane-1,5-diamine - a by-product of the adiponitrile synthesis (by hydrogenation of the secondary dinitrile obtained by adding HCN to the 2-position of 1,3-butadiene instead of the desired 1-position), which is cyclized to 3-methylpiperidine and dehydrogenated to 3-methylpyridine.
properties
3-Cyanopyridine is a white to amber colored solid that has a pungent odor. The compound dissolves in water and in many organic solvents and can be recrystallized from petroleum ether for purification.
use
Hydrogenation of nicotinonitrile in dilute acetic acid with Raney nickel as a catalyst with reaction termination after uptake of the theoretical amount of hydrogen gives nicotinaldehyde in 93% yield , a key component for the synthesis of the insecticide pymetrozine .
In a more recent process, pymetrozine is obtained in a one-pot reaction directly from 3-cyanopyridine and the aminotriazinone in the hydrogenation with Raney nickel in acetic acid in a 98% yield.
Complete hydrogenation of nicotinonitrile in dilute hydrochloric acid with palladium on activated carbon as a catalyst produces 3-aminomethylpyridine hydrochloride, which can be diazotized with ethyl nitrite and then boiled to form nicotinyl alcohol (3-pyridinemethanol) .
The reaction of nicotinonitrile with the Grignard compound n-propylmagnesium bromide produces 3-pyridyl-n-propyl ketone in 40% yield, which can be hydrogenated to 3-n-butylpyridine in a Wolff-Kishner reaction in 60% yield.
Synthesis of the vitamin B3 forms nicotinic acid (niacin) and nicotinamide (niacinamide)
By far the most important use of nicotinic acid nitrile is its conversion to nicotinic acid and in particular to nicotinic acid amide.
The complete chemical hydrolysis of 3-cyanopyridine with concentrated alkalis leads to sodium nicotinate, from which the free nicotinic acid is obtained in 97% yield and in high purity with hydrochloric acid.
The enzymatic hydrolysis with nitrilase provides pure nicotinic acid from nicotinonitrile with a conversion of up to 100%.
The mainly industrially used alternative routes to the chemical preparation of nicotinic acid - the oxidation of 3-picoline with concentrated nitric acid or with oxygen on a vanadium pentoxide contact or the oxidation of 2-methyl-5-ethylpyridine (from acetaldehyde and ammonia) with nitric acid - can are usually varied by the manufacturers depending on the availability and price of the raw materials.
The partial chemical hydrolysis of 3-cyanopyridine produces nicotinic acid amide, which usually contains 3–5% nicotinic acid as an impurity that is difficult to separate and which, when administered as a vitamin, can cause diarrhea in farm animals instead of supporting growth.
In contrast, the enzymatic hydrolysis with nitrile hydratase of immobilized cells of the wild type Rhodococcus rhodochrous J1 as a biocatalyst in multi-stage enzyme reactors with> 99.3% selectivity and 100% conversion yields highly pure nicotinamide. Product concentrations of 1.465 g / l can be achieved, the solid starting material nicotinonitrile being gradually solubilized during hydrolysis and the product (nicotinamide) crystallizing out at higher concentrations, i.e. H. the medium is solid at the beginning and at the end of the reaction.
Individual evidence
- ↑ a b W.M. Haynes: CRC Handbook of Chemistry and Physics, 93rd Edition . CRC Press, Boca Raton, FL, USA 2012, ISBN 978-1-4398-8049-4 , pp. 3-474 .
- ↑ a b c d e f g h Data sheet 3-Pyridinecarbonitrile from Sigma-Aldrich , accessed on May 10, 2017 ( PDF ).
- ↑ a b c 3-Cyanopyridine, Safety Data Sheet. (PDF; 150 kB) In: vertellus.com. Vertellus Holdings LLC, accessed May 11, 2017 .
- ↑ a b Patent US3644380 : Preparation of 3-cyanopyridine. Applied on November 24, 1969 , published February 22, 1972 , Applicant: Merck & Co., Inventor: R. Harmetz, RJ Tull.
- ↑ a b Entry on 3-cyanopyridines at TCI Europe, accessed on May 10, 2017.
- ↑ SM McElvain, MA Goese: The sulfonation of pyridine and the picolines . In: J. Am. Chem. Soc. tape 65 , no. 11 , 1943, pp. 2233-2236 , doi : 10.1021 / ja01251a063 .
- ↑ Patent US2409806 : Synthesis of nicotinic compounds. Filed December 8, 1941 , published October 22, 1946 , Applicant: Pittsburgh Coke & Chemical Co., Inventor: W. Shive, RA Glenn.
- ↑ MP Kaushik, V. Polshettiwar: N-octylquinolinium tribromide: A task specific quinoline based ionic liquid as a new Brominating agent . In: Ind. J. Chem. 45B, 2006, p. 2542-2545 ( res.in [PDF]).
- ↑ Patent US2491253 : Preparation of nicotinonitrile. Applied on August 9, 1944 , published December 13, 1949 , Applicant: American Cyanamide Co., Inventors: ML Crossley, VL King, EH Northey, TF Scholz.
- ↑ C. Yang, JM Williams: Palladium-catalyzed cyanation of aryl bromides promoted by low-level organotin compounds . In: Org. Lett. tape 6 , no. 17 , 2004, p. 2837-2840 , doi : 10.1021 / ol049621d .
- ↑ L. Yu et al .: Practical and scalable preparation of 2-methyleneglutaronitrile via an efficient and highly selective head-to-tail dimerization of acrylonitrile catalysed by low-loading of tricyclohexylphosphine . In: RSC Adv. Band 4 , 2014, p. 19122-19126 , doi : 10.1039 / C4RA02810D .
- ↑ A. Kagarlitskii, M. Iskakova, A. Turmukhambetov: Catalytic conversion of nicotine into nicotinonitrile - a pharmaceutical intermediate product . In: Pharm. Chem. J. Band 36 , no. 1 , 2002, p. 26-27 , doi : 10.1023 / A: 1015796724195 .
- ↑ VN Telvekar, RA Rane: A novel system for the synthesis of nitriles from carboxylic acids . In: Tetrahedron Lett. tape 48 , no. 34 , 2007, p. 6051-6053 , doi : 10.1016 / tetlett.2007.06.108 .
- ↑ S. Enthaler, M. Weidauer: Copper-catalyzed dehydration of primary amides to nitriles . In: Catalysis Lett. tape 141 , no. 8 , 2011, p. 1079-1085 , doi : 10.1007 / s10562-011-0660-9 .
- ↑ PC Teague, WA Short: Nicotinonitrile In: Organic Syntheses . 33, 1953, p. 52, doi : 10.15227 / orgsyn.033.0052 ; Coll. Vol. 4, 1963, p. 706 ( PDF ).
- ↑ Patent US5817827 : Method for the dehydration of amides to nitriles. Applied on October 22, 1996 , published October 6, 1998 , applicant: Hoffmann-La Roche Inc., inventor: W. Bonrath, H. Pauling.
- ↑ Nutrition - Niacin and Niacinamide. (PDF; 546 KB) In: ethorn.com. Lonza AG Consumer Care, accessed on May 20, 2017 .
- ↑ UN Kalevaru, BD Raju, VV Rao, A. Martin: ammoxidation of 3-picoline over V 2 O 5 / MgF 2 catalysts: Correlations between nicotinonitrile yield and O 2 and NH 3 chemisorption properties . In: Catalysis Commun. tape 9 , no. 5 , 2008, p. 715-720 , doi : 10.1016 / j.catcom.2007.08.009 .
- ↑ Patent US2510605 : Production of nitriles. Applied on April 6, 1946 , published June 6, 1950 , Applicants: Allied Chemical & Dye Corp., Inventors: F. Porter, M. Erchak, JN Cosby.
- ↑ Patent EP2305377A1 : Catalysts for the preparation of cyanopyridines and their use. Applied on September 29, 2009 , published on April 6, 2011 , applicant: Lonza Ltd., Polynt SA, inventors: A. Zenklusen, D. Pianzola, R. Laenza, G. Mazzoni, E. Armbruster, R. Chuck.
- ↑ UN Kalevaru, NN Madaan, A. Martin: Synthesis, characterization and catalytic performance of titania supported VPO catalysts for the ammoxidation of 3-picoline . In: Appl. Catalysis A: General . tape 391 , no. 1–2 , 2011, pp. 52-62 , doi : 10.1016 / j.apcata.2010.07.034 .
- ↑ a b Patent US5719045 : Process for preparing nicotinamide. Applied on October 31, 1996 , published on February 17, 1998 , applicant: Lonza AG, inventors: J. Heveling, E. Armbruster, L. Utiger, M. Rohner, H.-R. Dettwiler, RJ Chuck.
- ↑ Patent US7795169B2 : Process for preparing cyanopyridines and suitable catalysts therefor. Applied on July 30, 2004 , published on September 14, 2010 , applicant: Vertellus Specialties Inc., inventors: A. Fischer, A. Martin, B. Lucke, V. Kalevaru, C. Weckbecker, K. Huthmacher.
- ↑ Patent US5714610 : Process for the preparation of 3-methylpiperidine and 3-methylpyridine by catalytic cyclization of 2-methyl-1,5-diaminopentane. Applied March 30, 1994 , published February 3, 1998 , Applicant: Lonza Ltd., Inventor: J. Heveling, E. Armbruster, W. Siegrist.
- ↑ Patent US5646288 : Process for the preparation of aqueous nicotinaldehyde. Applied on June 2, 1995 , published on July 8, 1997 , applicant: Ciba-Geigy Corp., inventor: U. Siegrist, H. Szczepanski.
- ↑ Patent EP0613895B1 : Process for the preparation of 6-alkyl-4- (pyridin-3-yl-methylenamino) -4,5-dihydro-1,2,4-triazin-3 (2H) -one. Applied on February 21, 1994 , published on October 28, 1998 , Applicant: Novartis AG, Inventors: T. Pitterna, U. Siegrist, H. Szczepanski.
- ↑ Patent US2615896 : Preparation of 3-pyridyl-carbinol. Applied on January 20, 1950 , published October 28, 1952 , applicant: Hoffmann-La Roche Inc., inventor: GO Chase.
- ^ RL Frank, C. Weatherbee: Pyridines.III.3-n-butylpyridine and an unusual alkylation it its synthesis . In: J. Am. Chem. Soc. tape 70 , no. 10 , 1948, pp. 3482-3483 , doi : 10.1021 / ja01190a081 .
- ↑ Patent EP0242535A2 : Process for the production of coarsely crystalline nicotinic acid. Registered on February 24, 1987 , published on October 28, 1987 , applicant: Degussa AG, inventors: A. Möller, H. Friedrich, H. Kuhn, K. Winkler.
- ↑ CD Mathew, T. Nagasawa, M. Kobayashi, H. Yamada: Nitrilase-catalyzed production of nicotinic acid from 3-cyanopyridine in Rhodococcus rhodochrous J1 . In: Appl. Environ. Microbiol. tape 54 , no. 4 , 1988, pp. 1030-1032 , PMC 202591 (free full text).
- ↑ M. Baumann, IR Baxendale: An overview of the synthetic routes to the best selling drugs containing 6-membered heterocycles . In: Beilstein J. Org. Chem. Volume 9 , 2013, p. 2265-2319 , doi : 10.3762 / bjoc.9.265 ( beilstein-journals.org ).
- ↑ CF Krewson, JF Couch: The hydrolysis of nicotinonitrile by ammonia . In: J. Am. Chem. Soc. tape 65 , no. 11 , 1943, pp. 2256-2257 , doi : 10.1021 / ja01251a505 .
- ^ AS Bommarius, BR Riebel: Biocatalysis - Fundamentals and Applications . Wiley-VCH, Weinheim 2004, ISBN 978-3-527-30344-1 , pp. 162 .
- ↑ T. Nagasawa, CD Mathew, J. Mauger, H. Yamada: Nitrilase hydratase-catalyzed production of nicotinamide from 3-cyanopyridine in Rhodococcus rhodochrous J1 . In: Appl. Environ. Microbiol. tape 54 , no. 7 , 1988, pp. 1766–1769 , PMC 202743 (free full text).
- ↑ A. Liese, K. Seelbach, C. Wandrey (Eds.): Industrial Biotransformations, 2nd ed. Wiley-VCH, Weinheim 2006, ISBN 978-3-527-31001-2 , pp. 484 .