Copper (I) cyanide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| __ Cu + __ CN - | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Copper (I) cyanide | |||||||||||||||
| Ratio formula | CuCN | |||||||||||||||
| Brief description |
greenish-white to white powder with an unpleasant odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 89.56 g · mol -1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
2.92 g cm −3 (25 ° C) |
|||||||||||||||
| Melting point |
473 ° C |
|||||||||||||||
| solubility |
almost insoluble in water at 20 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
96.2 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Copper (I) cyanide is a greenish-white to white powder that is almost insoluble in water and melts at 473 ° C.
Extraction and presentation
Copper (I) cyanide can be produced in a redox reaction from copper sulfate CuSO 4 and sodium cyanide NaCN with the formation of dicyan (CN) 2 and sodium sulfate Na 2 SO 4 . The Cu 2+ ions are reduced to Cu + while some of the cyanide ions are oxidized to dicyan :
use
Copper (I) cyanide is used in electroplating . For copper plating and in the United brass ung in basic bath is made of copper (I) cyanide and sodium cyanide structure NACU (CN) 2 used.
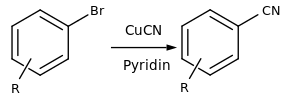
In organic chemistry, it is used as a reagent in the Rosenmund-von-Braun reaction for the production of aryl nitriles .
For the synthesis of organocuprates , copper (I) cyanide is reacted with organolithium compounds as part of a transmetallation reaction.
- Reaction of copper (I) cyanide and butyllithium (Bu = butyl )
Individual evidence
- ↑ Data sheet copper (I) cyanide from Acros, accessed on May 20, 2010.
- ↑ a b c d data sheet copper (I) cyanide (PDF) from Merck , accessed on May 20, 2010.
- ↑ Data sheet Copper (I) cyanide from Sigma-Aldrich , accessed on July 9, 2019 ( PDF ).
- ↑ a b Entry on copper (I) cyanide in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ Not explicitly listed in Regulation (EC) No. 1272/2008 (CLP) , but with the indicated labeling it falls under the group entry salts of hydrogen cyanide with the exception of complex cyanides such as ferrocyanides, ferricyanides and mercuric oxycyanide and those specified elsewhere in this Annex in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-19.
- ↑ JV Supniewski, PL Salzberg: Allyl cyanide In: Organic Syntheses . 8, 1928, p. 4, doi : 10.15227 / orgsyn.008.0004 ; Coll. Vol. 1, 1941, p. 46 ( PDF ).
- ↑ Walter Müller: Galvanic layers and their testing. Springer-Verlag, 2013, ISBN 978-3-322-90604-5 , p. 32 ( limited preview in the Google book search).