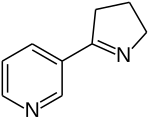
Myosmin
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Myosmin | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 9 H 10 N 2 | |||||||||||||||
| Brief description |
yellowish solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 146.19 g · mol -1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
42-44 ° C |
|||||||||||||||
| boiling point |
82–83 ° C (0.7 h Pa ) |
|||||||||||||||
| solubility |
soluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Myosmin is a chemical compound from the group of tobacco alkaloids . It is made up of a pyridine and a dihydro pyrrole ring.
Occurrence
Myosmin is found in tobacco plants . It could also be detected in the range of 0.2 to 2 ng / g in nuts and also in maize.
Manufacturing
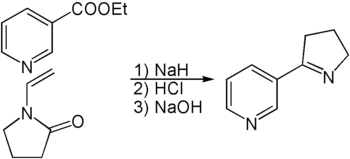
The synthesis of myosmin can be accomplished in several ways. One possibility is the reaction of an ester of nicotinic acid with the protected butyrolactam vinylpyrrolidone .
Biosynthetically, myosmin can be obtained by dehydrogenating nornicotine . Here it is an intermediate product of the breakdown of nornicotine to nicotinic acid. However, the fact that myosmin could also be detected in plants that do not produce nicotine suggests other previously unknown biosynthetic pathways.
properties
Myosmin is a yellowish solid with a melting range of 42 to 44 ° C, which slowly turns brown at room temperature.
use
Myosmin can be used to synthesize nornicotin . For this purpose, it is either hydrogen on palladium - activated carbon - catalyst or with sodium borohydride reduced .
Individual evidence
- ↑ a b c d data sheet Myosmin at AlfaAesar, accessed on March 17, 2010 ( PDF )(JavaScript required) .
- ↑ a b Myosmine data sheet at Sigma-Aldrich , accessed on October 18, 2016 ( PDF ).
- ↑ a b Susanna Glas: Autoradiographic studies on the toxicokinetics of myosmin in rats , dissertation, LMU Munich 2003. urn : nbn: de: bvb: 19-9589 .
- ↑ Svante Brandänge, Lars Lindblom u. a .: N-Vinyl as NH Protecting Group. A Convenient Synthesis of Myosmine. In: Acta Chemica Scandinavica. 30b, 1976, p. 93, doi : 10.3891 / acta.chem.scand.30b-0093 .
- ^ Paul G. Haines, Abner Eisner, CF Woodward: Chemical Reactivity of Myosmine. In: Journal of the American Chemical Society. 67, 1945, pp. 1258-1260, doi : 10.1021 / ja01224a011 .
- ^ TJ Dickerson, KD Janda; J. Am. Chem. Soc. 2002 , 124 , 13, 3220-3221; doi : 10.1021 / ja017774f , PMID 11916401 .