γ-butyrolactam
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | γ-butyrolactam | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 4 H 7 NO | ||||||||||||||||||
| Brief description |
colorless to light yellow solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 85.11 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.12 g cm −3 |
||||||||||||||||||
| Melting point |
25 ° C |
||||||||||||||||||
| boiling point |
245 ° C |
||||||||||||||||||
| Vapor pressure |
|
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.4853 |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
γ-butyrolactam , short γ-lactam or butyrolactam , is an organic-chemical compound from the group of lactams . Butyrolactam is the lactam of γ-aminobutyric acid ( GABA ), an inhibitory neurotransmitter , and it can be converted to GABA by hydrolysis .
Extraction and presentation
The large-scale production of γ-butyrolactam now takes place almost exclusively by reacting γ-butyrolactone with ammonia at temperatures of 250-290 ° C. and pressures of 4-14 bar on magnesium silicate contacts in the gas phase.
The reaction is carried out in tubular reactors in which the catalyst (magnesium silicate) is arranged in granulate form as a fixed bed . The yield is 75-85%. After a distillation , the desired γ-butyrolactam can be obtained in a purity of at least 99.5%.
Further synthesis variants, which are, however, carried out for economic or process engineering reasons, not industrially, the carbonylation of allyl amine , the reaction of maleic or succinic anhydride in aqueous ammonia in the presence of palladium - ruthenium - catalysts , the catalytic reduction of succinimide , or the hydrogenation of succinonitrile at Hydrolysis conditions.
In 2010, the estimated demand for γ-butyrolactam was around 32,000 tons. Important manufacturers of γ-butyrolactam are BASF and ISP ( International Specialty Products , now Ashland Inc. ).
properties
Butyrolactam is a solid that deliquesces at room temperature and melts at 25 ° C.
Safety-related parameters
Butyrolactam forms flammable vapor-air mixtures above the flash point of 138 ° C. The explosion range is between 1.8% by volume as the lower explosion limit (LEL) and 16.6% by volume as the upper explosion limit (UEL). The ignition temperature is 390 ° C. The substance therefore falls into temperature class T1.
use
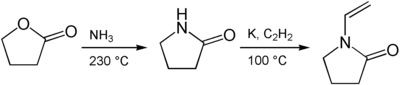
Butyrolactam is an intermediate in the production of polyvinylpyrrolidone . For this purpose, γ-lactone is first reacted with ammonia at 230 ° C to give butyrolactam, which in a further step reacts with elemental potassium and acetylene at 100 ° C to form N- vinylpyrrolidone .
It can also be used for the synthesis of N -methyl-2-pyrrolidone (NMP). For this purpose, it is reacted with methanol at 300 ° C. on aluminum oxide .
It is used as a high-boiling polar solvent in industrial processes .
Various derivatives of butyrolactam which are called racetams (e.g. Piracetam , Aniracetam , Oxiracetam , Pramiracetam and others) are used in medicine as a nootropic .
See also
Individual evidence
- ↑ a b c d e f g h i j k l m n Entry on 2-pyrrolidone in the GESTIS substance database of the IFA , accessed on January 20, 2020(JavaScript required) .
- ↑ R. Huisgen, H. Brade: The basicity constants of off-chain carboxylic acid amides in: Chem. Ber. , 1957 , 90 , pp. 1432-1436.
- ↑ Y. Uosaki, K. Sogo, T. Kunimine, T. Moriyoshi: Excess molar volumes of (a cyclic amide + water) at 298.15 K and 308.15 K , in: J. Chem. Thermodynamics , 1990 , 22 , p. 257 -262, doi : 10.1016 / 0021-9614 (90) 90196-W .
- ↑ a b c d Albrecht Ludwig Harreus, R. Backes, J.‐O. Eichler, R. Feuerhake, C. Jäkel, U. Mahn, R. Pinkos, R. Vogelsang: 2 ‐ Pyrrolidone. In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley ‐ VCH Verlag GmbH & Co. KGaA., October 15, 2011, p. 2, doi : 10.1002 / 14356007.a22_457.pub2 .
- ↑ W. Reppe: ethynylation VI , in: Justus Liebig's Ann. Chem. , 1955 , 596 , pp. 158-224.
- ^ W. Reppe: Vinylierung , in: Justus Liebigs Ann. Chem. , 1956 , 601 , pp. 81-138.
- ↑ Patent BASF DE 830194, 1951 .