Aniracetam
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Aniracetam | |||||||||||||||||||||
| other names |
1- (4-methoxybenzoyl) pyrrolidin-2-one ( IUPAC ) |
|||||||||||||||||||||
| Molecular formula | C 12 H 13 NO 3 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 219.24 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
121-122 ° C |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Aniracetam (trade name: Ampamet ® (I), manufacturer: Menarini ) is a drug similar to Piracetam from the group of nootropics that is used to change cognitive functions.
Presentation and extraction
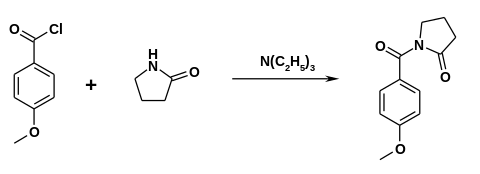
The active ingredient was developed by Hoffmann-La Roche in the 1970s . One synthesis is based on anisoyl chloride , which is reacted with 2-pyrrolidone in the presence of triethylamine .
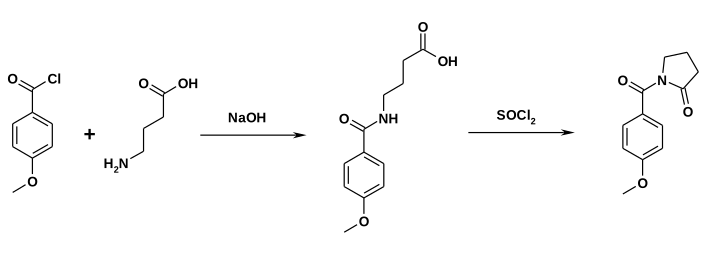
In an alternative synthesis, anisoyl chloride is first reacted with 4-aminobutyric acid. The ring closure then takes place by reaction with thionyl chloride .
Chemical properties
Aniracetam is white; it is mostly in the form of a crystalline powder, is odorless and has a bitter taste. The solubility in water is 1.065 g / L, in methanol 25 g / L, in acetonitrile 217 g / L, in chloroform 400 g / L.
dose
The therapeutic dose is usually 750 mg twice a day.
possible side effects
Individual evidence
- ^ The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals . 14th edition. 2006, ISBN 0-911910-00-X , p. 108.
- ↑ a b c data sheet aniracetam from Sigma-Aldrich , accessed on June 16, 2011 ( PDF ).
- ↑ Patent EP 5 143 Hoffmann-La Roche 1978.
- ↑ Patent EP 44 088 Hoffmann-La Roche 1978.
- ^ A b A. Kleemann, J. Engel, B. Kutscher, D. Reichert: Pharmaceutical Substances - Synthesis, Patents, Applications. 4th edition. Thieme 2001, ISBN 3-13-115134-X .