Nornicotine
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
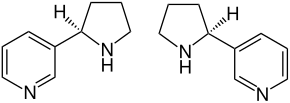
| ( R ) stereoisomer (left) and ( S ) stereoisomer (right) | ||||||||||
| General | ||||||||||
| Surname | Nornicotine | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 9 H 12 N 2 | |||||||||
| Brief description |
yellow liquid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 148.20 g · mol -1 | |||||||||
| Physical state |
liquid |
|||||||||
| density |
1.0737 (19 ° C ) |
|||||||||
| boiling point |
|
|||||||||
| solubility |
soluble in water (50 mg ml −1 ) |
|||||||||
| Refractive index |
1.5378 (18 ° C) |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||
Nornicotine is a chemical compound from the group of alkaloids . Their structure is derived from that of nicotine , which also has a methyl group on the nitrogen atom of the pyrrolidine ring .
Occurrence
Nornicotine occurs naturally in some types of tobacco . Here it is formed by the enzymatic demethylation of nicotine. As a metabolite of nicotine, it is also found in the blood and urine of smokers and laboratory animals.
Manufacturing
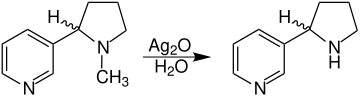
Several routes can be followed for the synthesis of ( RS ) -nornicotine. This includes the demethylation of ( RS ) nicotine. The methyl group can be split off, for example, by reacting with silver oxide :
The reduction of 3-myosmin, for example with molecular hydrogen on a palladium - activated carbon - catalyst or with sodium borohydride gives ( RS ) -nornicotine in moderate to good yield:
Analytics
The reliable identification and quantification of nornicotine and many other nicotine metabolites in various test materials is possible after adequate sample preparation by coupling the HPLC with mass spectrometry
properties
Nornicotine is a yellow liquid. It is a chiral compound made up of a pyridine and a pyrrolidine ring. So there are two stereoisomers , ( R ) -nornicotine and ( S ) -nornicotine. By far the most important stereoisomer is ( S ) -nornicotine, which is often just called nornicotine. ( S ) -Nornicotine has the same configuration as natural nicotine . ( R ) -Nornicotine and ( RS ) -Nornicotine [synonyms: rac -Nornicotine and (±) -Nornicotine] are of little importance.
Reactions
Nornicotine can be used to synthesize nicotine. The reaction can be carried out under the action of a base with methyl iodide or in a Leuckart-Wallach reaction with formaldehyde and formic acid .
Individual evidence
- ↑ a b c d e data sheet (±) -Nornicotine from Sigma-Aldrich , accessed on April 16, 2011 ( PDF ).
- ^ The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals. 14th edition. 2006, ISBN 0-911910-00-X , p. 1160.
- ↑ a b c David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-452.
- ↑ Entry on nornicotine. In: Römpp Online . Georg Thieme Verlag, accessed on July 31, 2015.
- ↑ J. Mao, Y. Xu, B. Lu, J. Liu, G. Hong, Q. Zhang, S. Sun, J. Zhang: Simultaneous determination of nicotine and its nine metabolites in rat blood utilizing microdialysis coupled with UPLC- tandem mass spectrometry for pharmacokinetic application. In: Anal Bioanal Chem. 407 (14), May 2015, pp. 4101-4109. PMID 25824453 .
- ↑ K. Rangiah, WT Hwang, C. Mesaros, A. Vachani, IA Blair: Nicotine exposure and metabolizer phenotypes from analysis of urinary nicotine and its 15 metabolites by LC-MS. In: Bioanalysis. 3 (7), Apr 2011, pp. 745-761. PMID 21452992 .
- ↑ a b Ernst Späth, Friederike Kesztler: About the occurrence of d, l -nor-nicotine, d, l -anatabine and l -anabasine in tobacco (XII. Communication on tobacco alkaloids) . In: Reports of the German Chemical Society . tape 70 , no. 4 , April 7, 1937, pp. 704-709 , doi : 10.1002 / cber.19370700422 .
- ^ Paul G. Haines, Abner Eisner, CF Woodward: Chemical Reactivity of Myosmine . In: Journal of the American Chemical Society . tape 67 , no. 8 , May 1, 2002, pp. 1258-1262 , doi : 10.1021 / ja01224a011 .
- ^ TJ Dickerson, KD Janda: Aqueous aldol catalysis by a nicotine metabolite. In: J. Am. Chem. Soc. 124, 13, 2002, pp. 3220-3221. PMID 11916401 .
- ↑ LB von Weymarn, NM Thomson, EC Donny, DK Hatsukami, SE Murphy: Quantitation of the Minor Tobacco Alkaloids Nornicotine, Anatabine, and Anabasine in Smokers' Urine by High Throughput Liquid Chromatography-Mass Spectrometry. In: Chem Res Toxicol. 29 (3), Mar 21, 2016, pp. 390-397. PMID 26825008 .
- ↑ J. Mao, Y. Xu, B. Lu, J. Liu, G. Hong, Q. Zhang, S. Sun, J. Zhang: Simultaneous determination of nicotine and its nine metabolites in rat blood utilizing microdialysis coupled with UPLC- tandem mass spectrometry for pharmacokinetic application. In: Anal Bioanal Chem. 407 (14), May 2015, pp. 4101-4109. PMID 25824453 .
- ↑ Sandrine Girard, Richard J. Robins, Jean Villiéras, Jacques Lebreton: A short and efficient synthesis of unnatural (R) -nicotine . In: Tetrahedron Letters . tape 41 , no. 48 , November 25, 2000, pp. 9245-9249 , doi : 10.1016 / S0040-4039 (00) 01675-0 ( PDF ).