Anatabine
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
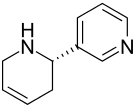
|
||||||||||
| General | ||||||||||
| Surname | Anatabine | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 10 H 12 N 2 | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 160.22 g · mol -1 | |||||||||
| density |
1.09 g cm −3 (19 ° C) |
|||||||||
| boiling point | ||||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Anatabine is a heterocyclic chemical compound from the group of tobacco alkaloids , more precisely the tobacco secondary alkaloids. It is made up of a pyridine and a tetrahydropyridine ring.
Occurrence
Anatabine is a natural substance that is formed in tobacco plants, among other things. The concentration of anatabine is strongly dependent on the type of tobacco examined, but is usually higher than that of the secondary alkaloids anabasine , 2,3′-bipyridine , myosmin and nicotyrine .
properties
Anatabine is a colorless to yellowish liquid. It is a chiral compound with a boiling point of 145 to 146 ° C at 13.3 hPa. Only the ( S ) enantiomer of anatabine is formed biosynthetically . The ( R ) -enantiomer is only accessible synthetically .
Reactions
Another secondary tobacco alkaloid, anabasine , can be produced from anatabine by reduction . This can, for example, molecular hydrogen on palladium - activated carbon - catalyst happen.
Individual evidence
- ↑ a b Ernst Späth, Friederike Kesztler: L -Anatabin, a new tobacco alkaloid (XI. Communication on tobacco bases). In: Reports of the German Chemical Society. 70, 1937, p. 239, doi : 10.1002 / cber.19370700215 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Careen Merckel, Fritz Pragst : Analysis of cigarettes on additives , Institute of Forensic Medicine, Charité Berlin, 2005..
- ↑ E. Broad Maier: Alkaloids: narcotics, hallucinogens and other drugs, lead compounds from nature , Vieweg + Teubner Verlag, 3rd edition, 2008, p 31: ISBN 3-8348-0531-9 .
- ↑ C.-M. Yang and DD. Tanner: A simple synthesis of (±) -1,2,3,6-tetrahydro-2,3′-bipyridine (anatabine) and (±) -3- (2-piperidinyl) pyridine (anabasine) from lithium aluminum hydride and pyridines . In: Canadian Journal of Chemistry . 75 (6), 1997, pp. 616-620, doi : 10.1139 / v97-073 .
literature
- E. Leete (1975): Biosynthesis of anatabine and anabasine in Nicotiana glutinosa . J. Chem. Soc., Chem. Commun. 9-10, doi : 10.1039 / C39750000009 .
- E. Leete and SA. Slattery (1976): Incorporation of [2- 14 C] and [6- 14 C] Nicotinic Acid into the Tobacco Alkaloids. Biosynthesis of Anatabine and α, β-Dipyridyl . In: J Am Chem Soc. 98 (20): 6326-30; doi : 10.1021 / ja00436a043 . PMID 965646 .
