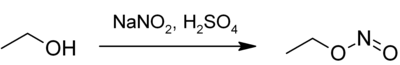Ethyl nitrite
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Ethyl nitrite | |||||||||||||||
| other names |
Ethyl nitrous acid |
|||||||||||||||
| Molecular formula | C 2 H 5 NO 2 | |||||||||||||||
| Brief description |
colorless to yellowish gas or liquid with an ethereal odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 75.07 g · mol -1 | |||||||||||||||
| Physical state |
gaseous |
|||||||||||||||
| density |
0.9062 g cm −3 (liquid at 8.8 ° C) |
|||||||||||||||
| Melting point |
−50 ° C |
|||||||||||||||
| boiling point |
17 ° C |
|||||||||||||||
| solubility |
little in water with decomposition |
|||||||||||||||
| Refractive index |
1.3418 (10 ° C, 589 nm) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Ethyl nitrite , the nitrous acid ester of ethanol , is a colorless, extremely flammable and toxic gas at room temperature (20 ° C).
presentation
Ethyl nitrite is formed when cold, dilute sulfuric acid acts on a water- ethanol solution of sodium nitrite .
Ethyl nitrite can also be produced by reacting silver nitrite with iodoethane .
properties
Physical Properties
With a boiling point at normal pressure of 17 ° C, ethyl nitrite is a colorless to yellowish liquid with an ethereal odor at normal temperature, and a colorless gas at room temperature . The compound has a melting point of -50 ° C . The vapor pressure function results according to August according to lg (P) = −A / T + B (P in Torr, T in K) with A = 1340 and B = 7.50 in the temperature range from 255 K to 280 K. From the vapor pressure function one can a molar enthalpy of vaporization of 27.84 kJ mol −1 can be derived.
Like other alkyl nitrites, ethyl nitrite is a mixture of the anti and syn conformer . The enthalpy of conversion between the two conformers is 1.477 kJ mol −1 .
Safety-related parameters
Ethyl nitrite forms highly flammable vapor-air mixtures. The compound has a flash point of −35 ° C. The explosion range is between 3.0% by volume (90 g / m 3 ) as the lower explosion limit (LEL) and 50% by volume (1555 g / m 3 ) as the upper explosion limit (UEL). The maximum explosion pressure is 11.6 bar. The limit gap width was determined to be 0.96 mm. This results in an assignment to explosion group IIA. The ignition temperature is 90 ° C. The substance therefore falls into temperature class T6.
use
Ethyl nitrite is the main ingredient in Witdulsies , a traditional, ethanol-based South African remedy for colds and flu that was sold in pharmacies. However, the FDA banned the drug in 1980 in the USA, marketed as sweet nitrite or saltpeter spirit.
Individual evidence
- ↑ a b c d e f g h i j k Entry on ethyl nitrite in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ Eric G. Cowley, James R. Partington: 293. Studies in dielectric polarization. Parts VIII, IX, X, and XI . In: Journal of the Chemical Society (Resumed) . 1933, ISSN 0368-1769 , p. 1252 , doi : 10.1039 / jr9330001252 .
- ↑ Entry on Ethyl nitrite in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers and / or distributors can expand the harmonized classification and labeling .
- ↑ LF Fieser, M. Fieser: Organic chemistry. 2nd edition, Verlag Chemie, 1975. - P. Tarte: Rotational Isomerism as a General Property of Alkyl Nitrites . In: The Journal of Chemical Physics . tape 20 , no. 10 , 1952, pp. 1570-1575 , doi : 10.1063 / 1.1700218 .
- ↑ J. Thiele and H. Eichwede, Justus Liebigs Annalen der Chemie 311 (1900), footnote on page 366: Somewhat different from the usual methods, the same can be presented advantageously by dissolving 200 g of sodium nitrite in so much water that 150 g Do not precipitate alcohol and allow dilute hydrochloric acid to run into the mixture at ordinary temperature, the ethyl nitrite developing in a regular stream. Yield 80-85 pC. the theory.
- ^ Donald L. Pavia, Gary M. Lampman, George S. Kriz: Organic Chemistry , Volume 2. Thompson Custom Publishing, Mason, Ohio 2004, ISBN 0-03-014813-8 .
- ↑ a b H. W. Thompson, FS Dainton: The photochemistry of alkyl nitrites. III . In: Transactions of the Faraday Society . tape 33 , 1937, pp. 1546-1555 , doi : 10.1039 / TF9373301546 .
- ↑ AI Lazaar, SH Bauer: Intramolecular conversion rates over low barriers. 2. The alkyl nitrites . In: The Journal of Physical Chemistry . tape 88 , no. 14 , 1984, pp. 3052-3059 , doi : 10.1021 / j150658a025 .
- ^ A b c d E. Brandes, W. Möller: Safety-related parameters - Volume 1: Flammable liquids and gases , Wirtschaftsverlag NW - Verlag für neue Wissenschaft GmbH, Bremerhaven 2003.
- ↑ FDA: Rulemaking History for OTC Sweet Spirits of Nitre Drug Products