Cholesterol biosynthesis
The cholesterol is the pathway that eukaryotes capable cholesterol produce from simple starting materials. Cholesterol is of paramount importance as a component of the cell membrane , as a storage lipid and as the basis for the synthesis of other important substances.
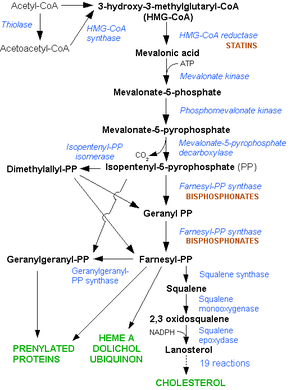
The starting point for cholesterol synthesis is dimethylallyl pyrophosphate (DMAPP), which is produced in the mevalonate pathway . The biosynthesis of cholesterol from DMAPP takes place via 18 intermediate stages.
In humans, the liver and the intestinal lining are the main sites of cholesterol synthesis. Many cells in the human organism are able to synthesize cholesterol. In terms of quantity, however, most of it is produced by the liver. The brain completely synthesizes the cholesterol it needs because it can not cross the blood-brain barrier .
Reaction steps
The biosynthesis of cholesterol starts from the end products of the mevalonate biosynthetic pathway , from dimethylallyl pyrophosphate and isopentenyl pyrophosphate .
GPP and farnesyl pyrophosphate
DMAPP and IPP are linked by a head-to-tail condensation by the GGPP synthase to form geranyl pyrophosphate ( GPP ), the same enzyme then links another IPP with the GPP to form farnesyl pyrophosphate (FPP ) by a further head-to-tail condensation ).
Squalene
 + 2 NADP H / H + + 2 NADP + + PP i
+ 2 NADP H / H + + 2 NADP + + PP i 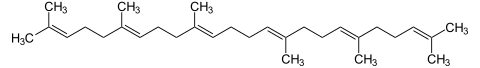
Two FPPs are linked in a tail-to-tail condensation via the intermediate compound praesqualene pyrophosphate with the aid of squalene synthase to form squalene .
Epoxysqualen
Squalene reacts with squalene monooxygenase to form (S) -2,3-epoxysqualene.
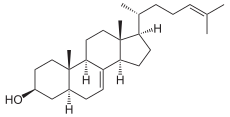
Lanosterol
Cycled Epoxysqualen using the lanosterol synthase , a Protosterol cation to lanosterol .
Lanosterol is converted to cholesterol via various intermediate compounds; this entire enzymatic process is localized in the membrane of the endoplasmic reticulum .
4,4-dimethyl-5α-cholesta-8,14,24-trien-3β-ol
The enzyme lanosterol demethylase catalyzes the dehydrogenative demethylation of lanosterol to 4,4-dimethyl-5α-cholesta-8,14,24-trien-3β-ol.
 + 3 O 2 + 3 NADPH + formate + 4 H 2 O + 3 NADP +
+ 3 O 2 + 3 NADPH + formate + 4 H 2 O + 3 NADP +
In several oxidation steps, the 14-methyl group of the lanosterol is removed and a 14,15 double bond is created, with formate being split off.
14-demethyllanosterol
4,4-Dimethyl-5α-cholesta-8,14,24-trien-3β-ol is hydrogenated using sterol-Delta14 reductase , resulting in 14-demethyllanosterol.
4α-methylzymosterol-4-carboxylate or zymosterol carboxylate
The oxidation of the 4-methyl group on 14-demethyllanosterol or on 4α-methyl-zymosterol is catalyzed by 4-methylsterol monooxygenase . The result is 4α-methyl-zymosterol-4-carboxylate or zymosterol carboxylate.
4-methylzymosterone or zymosterone
 + NADP + + CO 2 + NADP H / H +
+ NADP + + CO 2 + NADP H / H + 
4alpha-methylzymosterol-4-carboxylate is converted to 3-keto-4-methylzymosterol with the help of sterol-4alpha-carboxylate-3-dehydrogenase . Alternatively, the enzyme accepts zymosterol carboxylate as a substrate; this creates zymosterone.
4α-methylzymosterol or zymosterol
The enzyme 3-ketosteroid reductase (HSD17B7) reduces zymosterone to zymosterol. Alternatively, it is able to reduce 4α-methylzymosterone to 4α-methylzymosterol.
Zymosterone is hydrogenated to zymosterol.
5α-Cholesta-7,24-dien-3β-ol
Zymosterol is rearranged to 5α-Cholesta-7,24-dien-3β-ol, catalyzed by the sterol delta8 / 7 isomerase .
Lathosterol
5α-Cholesta-7,24-dien-3β-ol is hydrogenated to Lathosterol using sterol-Delta24 reductase .
7-dehydrocholesterol or desmosterin
 + O 2 + NADP H / H + + 2 H 2 O + NADP +
+ O 2 + NADP H / H + + 2 H 2 O + NADP + 
Lathosterol is dehydrated to 7-dehydrocholesterol with the help of lathosterol oxidase .
Alternatively, 5α-Cholesta-7,24-dien-3β-ol is hydrogenated to desmosterin using 7-dehydrocholesterol reductase .
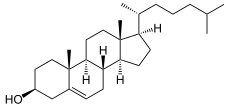
cholesterol
7-dehydrocholesterol is hydrogenated to cholesterol. The catalyst is 7-dehydrocholesterol reductase .
Desmosterin is hydrogenated to cholesterol, catalyzed by the sterol Delta24 reductase .
history
Konrad Bloch and Feodor Lynen shared the Nobel Prize in Physiology / Medicine in 1964 for the discovery of cholesterol and fatty acid metabolism and its regulation. Other important contributions to the elucidation of the biosynthesis of cholesterol were made by George Joseph Popják and John W. Cornforth .
Individual evidence
- ^ Information from the Nobel Foundation on the 1964 award ceremony to Konrad Bloch and Feodor Lynen (English).
Web links
- D'Eustachio / reactome: cholesterol biosynthesis