Phosphofructokinase 1
| Phosphofructokinase 1 | ||
|---|---|---|

|
||
| Mass / length primary structure | 780 amino acids | |
| Secondary to quaternary structure | Tetramer MMMM, LLLL, MMLL etc. | |
| Cofactor | Mg 2+ | |
| Isoforms | L1, L2, M1, M2, P | |
| Identifier | ||
| Gene name (s) | PFKM , PFKL , PFKP | |
| External IDs |
|
|
| Enzyme classification | ||
| EC, category | 2.7.1.11 , kinase | |
| Response type | Phosphorylation | |
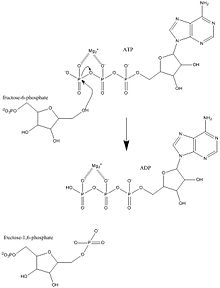
| Substrate | ATP + D- fructose-6-phosphate | |
| Products | ADP + D- fructose-1,6-bisphosphate | |
| Occurrence | ||
| Parent taxon | Creature | |
Phosphofructokinase 1 , abbreviated PFK1 or PFK , also fructose-6-phosphate-kinase is an enzyme that catalyzes the rate-limiting step of glycolysis , the conversion of fructose-6-phosphate to fructose-1,6-bisphosphate . Phosphofructokinase has a decisive influence on how much available energy the cell ( ATP , citrate , NADH / H + ) has. It occurs in all living things. There are five isoforms in humans that are produced by three different genes : PFKM (muscle), PFKL (liver), and PFKP (platelets). Mutations in PFKM are the cause of the rare Tarui disease .
structure
The pFK1 from mammals is a 340 kDa heavy tetramer consisting of various combinations of three types of subunits: Muscle (M), liver (L) and platelet (P). The composition of the PFK1 tetramer differs depending on the type of tissue in which it is present. For example , a mature muscle expresses only the M isozyme, so the PFK1 of the muscle consists exclusively of homotetramers of M4. The liver and kidneys predominantly express the L isoform. In erythrocytes, both M and L subunits tetramerize randomly to form M4 as well as L4 and the three hybrid forms of the enzyme (ML3, M2L2, M3L). As a result, the kinetic and regulatory properties of the various isoenzymes depend on the composition of the subunits. Tissue-specific changes in PFK activity and isoenzymic content contribute significantly to the variety of glycolysis and gluconeogenesis rates observed for different tissues.
PFK1 is an allosteric enzyme and has a structure similar to hemoglobin if it is a dimer of a dimer. Each subunit of the tetramer consists of 319 amino acids and consists of two domains. One domain contains the ATP binding site, the other half the substrate binding site (fructose-6-phosphate, F6P) and a separate allosteric binding site. Each domain is a β-barrel and has a cylindrical β-sheet surrounded by α-helices .
On the opposite side of each subunit from each active site, the allosteric site is at the interface between the subunits in the dimer. ATP and AMP compete for this binding site. The N -terminal domain has a catalytic role, which binds the ATP, and the C -terminal domain has a regulatory role.
mechanism
Fructose-6-phosphate acts as an allosteric activator. PFK1 prefers the β-anomer of fructose-6-phosphate. When it binds, fructose-6-phosphate induces a reactive conformation that orients both itself and ATP for base-catalyzed phosphate transfer. Fructose-6-phosphate is activated as a nucleophile when the hydroxy group on the C1 atom is deprotonated by Asp128 . The oxygen nucleophile removes the γ-phosphate from ATP and forms ADP. Asp128 is then deprotonated. Kinetic studies suggest that Asp128 must be protonated for the reverse reaction. Crystal structures show a water molecule in the active center. This is supposed to facilitate the transfer of protons from and to Asp128.
PFK1 is an allosteric enzyme whose activity can be described with the symmetry model of allostery , with a concerted transition from an enzymatically inactive T-state ( tense state ) to the active R-state ( relaxed state ). F6P binds with high affinity to the R-state enzyme, but not to the T-state enzyme. For each F6P molecule that binds to PFK1, the enzyme progressively switches from the T state to the R state. A graph in which the PFK1 activity is plotted against increasing F6P concentrations would thus assume the sigmoid curve which is traditionally associated with allosteric enzymes.
PFK1 belongs to the family of phospho transferases and catalyzes the transfer of γ-phosphate of ATP to fructose-6-phosphate. The active center includes both ATP, Mg 2+ and the F6P binding sites. Allosteric activators such as AMP and ADP bind to the allosteric site to facilitate the formation of the R-state by inducing structural changes in the enzyme. Similarly, inhibitors such as ATP and PEP bind to the same allosteric site and facilitate the formation of the T-state, thereby inhibiting enzyme activity.
Some suggested residues involved in substrate binding of PFK1 in E. coli include Asp127 and Arg171 .
In the PFK1 of Geobacillus stearothermophilus the positively charged side chain of the Arg162 residue forms a hydrogen-bonded salt bridge with the negatively charged phosphate group of F6P, an interaction that stabilizes the R state compared to the T state and partly for the homotropic effect of F6P -Binding is responsible. In the T state, the enzyme conformation shifts slightly, so that the space previously occupied by Arg162 is replaced by Glu161 . This swapping of positions between adjacent amino acid residues inhibits the ability of F6P to bind the enzyme.
Position in the energy metabolism
The central role of glycolysis in the energy metabolism is based on the fact that the substrate for this process comes from very different degradation pathways, which are thus brought together. The further breakdown in glycolysis ultimately releases energy that is used to synthesize high-energy ATP . The turnover in glycolysis is regulated by inhibiting the enzymes involved . In addition to the reaction of phosphofructokinase (PFK), that of pyruvate kinase and others would also be conceivable . Today, however, the most important control point is the PFK, which catalyzes the conversion of fructose-6-phosphate into fructose-1,6-bisphosphate . PFK is inhibited by higher ATP concentrations ; if one considers glycolysis as a whole, then this can be interpreted as an end product inhibition of glycolysis. At the enzyme kinetic level , the inhibition by ATP means that the Michaelis-Menten constant (K m value) of the PFK increases by ATP.
These properties of PFK are the most important aspect of molecular explanations of the Pasteur effect , whereby when switching from anaerobic to aerobic cell metabolism at a constant power state metabolites power is throttled in the glycolysis.
Regulator functions
Regulation in the cell
PFK1 has its catalytic center at the N terminus, the regulatory center at the C terminus of a fusion protein produced by gene duplication . As a result, both halves show sequence homologies , but were subject to separate optimization processes according to their task:
- the catalytic part binds the substrates fructose-6-phosphate (F-6-P) and ATP;
- At higher concentrations, ATP also occupies a (low-affinity) binding site on the regulatory part and acts from there as an allosteric inhibitor (" enzyme inhibition "). It shares an inhibitory function with other endogenous energy excess signals of the cell (NADH / H + and citrate). If, however, there are energy deficiency signals (AMP, ADP ), the enzyme is activated allosterically . As long as AMP and ADP prevail, they determine what happens.
- In erythrocytes , the intermediate 2,3-diphosphoglycerate formed in the Rapoport-Luebering cycle by the enzyme bisphosphoglycerate mutase acts as an inhibitor of phosphofructokinase.
The activity of phosphofructokinase 1 in muscle cells is also influenced by the pH value . A low pH inhibits the enzyme and slows down glycolysis. This happens, for example, when the muscles are strained and a lot of lactic acid is produced. This lowers the pH value in the cells.
Regulation in the organism
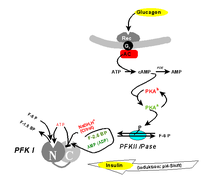
It has long been known that PFK1 can not only be inhibited by one of its substrates (ATP), but can also be activated in vitro by one of its products (F-1,6-BP) ("wrong enzyme"). The latter effect presumably does not occur in the cell, since F-1,6-BP never reaches the required equilibrium concentration due to aldolase activity . However, one isomeric molecule, fructose-2,6-bisphosphate (F-2,6-BP), has been found to be a physiological allosteric activator. F-2,6-BP conveys hunger signals ( blood sugar that is too low ), which are sent out by the body via glucagon or adrenaline . Like a "third messenger", it is used for propagation along the signal transduction chain glucagon - cAMP - PKA (see " second messenger ").
F-2,6-BP is the product of another specialized phosphofructokinase (PFKII). This " PFKII ", a fusion protein of phosphofructokinase and fructose-2,6-bisphosphatase in vertebrates , is one of the interconvertible enzymes, i. H. their activity is regulated by protein kinase A (PKA) and thus indirectly by hormonal signals: phosphorylation of a single serine residue switches off the kinase activity, while at the same time the phosphatase activity is switched on. The signal emanating from glucagon means that F-2,6-BP is no longer available. This brings the metabolite stream of glycolysis to a standstill at the PFKI. In the liver, the resulting G-6-P congestion is broken down by conversion into glucose (or the glycolysis reversed by gluconeogenesis ), which can be released into the bloodstream as a neutral molecule. The glucagon signal " too low blood sugar " is answered.
The opposite (insulin) signal "blood sugar too high" is evidently realized by an extremely pH-dependent activity profile. As an antagonist of the glucagon, the insulin also has an effect on the F-2,6-BP concentration by activating a phosphodiesterase to lower the cAMP level and to activate a phosphatase. This dephosphorylates the PFKII so that its kinase activity comes into play and F-2,6-BP is produced, which has an activating effect on the PFKI and thus the glycolysis. In this way, the excess blood glucose that triggers the signal is broken down. The activation of the PFK1 not only includes conformational changes of the individual subunits, but also the formation of aggregates to form higher oligomers.
In muscle cells, phosphorylation of the PFKII does not have an inhibitory effect on glycolysis, since isoenzymes are formed here, the regulation of which proceeds in the opposite direction. This is the basis of the Cori cycle , via which incompletely oxidized lactate from glycolysis is brought via the blood to the liver during muscle activity , where it is fed to gluconeogenesis (despite the same hormonal situation). In muscle cells, instead of glucagon, adrenaline primarily has a regulating function.
The isoenzyme in skeletal muscle does not have any phosphorylation sites for the PKA that allow regulation via phosphorylation by hormones. Therefore, adrenaline does not inhibit glycolysis in the skeletal muscle and thus does not inhibit the utilization of glucose, i.e. the energy production of the cells.
In the heart muscle, on the other hand, phosphorylation takes place. However, this has the effect of stimulating the kinase activity. So adrenaline causes an increase in the F-2,6-BP concentration and thus additionally stimulates glycolysis.
Phosphofructokinase in photosynthesis
During photosynthesis , light energy in plants produces ATP and NADP H / H + for biosynthesis. At the same time, carbon dioxide fixation ( assimilation ) in C 3 plants produces 3-phosphoglycerate (3-PG), an intermediate in both glycolysis and glucose biosynthesis (gluconeogenesis). If there is an excess of energy, the latter path is required, which ultimately leads to energy storage strength . Availability of 3-PG regulates (inhibits) PFKII, whereby gluconeogenesis is switched on and glycolysis is switched off.
- Excess energy signals from the cell (ATP, citrate and NADH / H + in animal, 3-PG in plant tissues) generally prevent the formation of superfluous ATPs.
Clinical significance
A genetic mutation in the PFKM gene leads to Tarui disease, a glycogen storage disease in which the ability of certain cell types to use carbohydrates as an energy source is impaired.
Tarui disease is a glycogen storage disorder that has symptoms such as muscle weakness ( myopathy ), movement-induced cramps and spasms, myoglobinuria (the presence of myoglobin in the urine, which indicates injury to the striated and heart muscles), and compensated hemolysis . ATP is a natural allosteric inhibitor of PFK to prevent unnecessary production of ATP through glycolysis. However, a mutation in Asp (543) Ala can lead to a stronger inhibitory effect of ATP (due to increased binding to the inhibitory allosteric binding site of PFK).
In order for cancer cells to meet their energy requirements due to their rapid cell growth and division, they survive more effectively if they have a hyperactive phosphofructokinase 1. When cancer cells grow and divide quickly, they may not have a lot of blood to begin with and therefore may be hypoxic (lack of oxygen). This triggers the O -GlcNAcylation of Ser529 and gives cancer cells a selective growth advantage.
Some viruses, including HIV , HCMV and Mayaro, affect cellular metabolic pathways such as glycolysis by increasing the activity of PFKs depending on the MOI . Herpes simplex viruses (HSV) increase the PFK activity by phosphorylating the enzyme on the serine residues. The HSV-1-induced glycolysis increases the ATP content, which is crucial for the replication mechanism of the virus.
See also
Individual evidence
- ↑ UniProt P08237
- ↑ Jeremy M. Berg, John L. Tymoczko, Lubert Stryer: Biochemistry . 6th edition. WH Freeman, 2007, ISBN 978-0-7167-8724-2 ( limited preview in Google Book Search).
- ↑ GA Dunaway, TP Kasten, T. Sebo, R. Trapp: Analysis of the phosphofructokinase subunits and isoenzymes in human tissues. In: The Biochemical journal. Volume 251, Number 3, May 1988, pp. 677-683, doi : 10.1042 / bj2510677 , PMID 2970843 , PMC 1149058 (free full text).
- ↑ PR Evans, GW Farrant, PJ Hudson: phosphofructokinase: structure and control. In: Philosophical transactions of the Royal Society of London. Series B, Biological sciences. Volume 293, Number 1063, June 1981, pp. 53-62, doi : 10.1098 / rstb.1981.0059 , PMID 6115424 .
- ↑ Y. Shirakihara, PR Evans: Crystal structure of the complex of phosphofructokinase from Escherichia coli with its reaction products. In: Journal of molecular biology. Volume 204, Number 4, December 1988, pp. 973-994, doi : 10.1016 / 0022-2836 (88) 90056-3 , PMID 2975709 .
- ↑ K. Banaszak, I. Mechin, G. Obmolova, M. Oldham, SH Chang, T. Ruiz, M. Radermacher, G. Kopperschläger, W. Rypniewski: The crystal structures of eukaryotic phosphofructokinases from baker's yeast and rabbit skeletal muscle. In: Journal of molecular biology. Volume 407, number 2, March 2011, pp. 284-297, doi : 10.1016 / j.jmb.2011.01.019 , PMID 21241708 .
- ^ Sophie T. Williams, Alex Gutteridge, Craig Porter, Gemma L. Holliday, Morwenna Hall: Phosphofructokinase I. In: Mechanism and Catalytic Site Atlas. EMBL-EBI, accessed on October 26, 2019 .
- ↑ K. Peskov, I. Goryanin, O. Demin: Kinetic model of phosphofructokinase-1 from Escherichia coli. In: Journal of bioinformatics and computational biology. Volume 6, Number 4, August 2008, pp. 843-867, PMID 18763746 .
- ↑ HW Hellinga, PR Evans: Mutations in the active site of Escherichia coli phosphofructokinase. In: Nature . Volume 327, Number 6121, 1987 Jun 4-10, pp. 437-439, doi : 10.1038 / 327437a0 , PMID 2953977 .
- ^ H. Robert Horton, Laurence A. Moran, K. Gray Scrimgeour, Marc D. Perry, J. David Rawn, Carsten Biele (translator): Biochemie. Pearson study. 4th updated edition, 2008, ISBN 978-3-8273-7312-0 , p. 468.
- ↑ a b c Joachim Rassow , Karin Hauser, Roland Netzker, Rainer Deutzmann: Dual series: Biochemistry . 2nd Edition. Thieme Verlag, Stuttgart 2008, ISBN 978-3-13-125352-1 , p. 219 f .
- ↑ H. Nakajima, N. Raben, T. Hamaguchi, T. Yamasaki: Phosphofructokinase deficiency; past, present and future. In: Current molecular medicine. Volume 2, Number 2, March 2002, pp. 197-212, doi : 10.2174 / 1566524024605734 , PMID 11949936 (review).
- ↑ A. Brüser, J. Kirchberger, T. Schöneberg: Altered allosteric regulation of muscle 6-phosphofructokinase causes Tarui disease. In: Biochemical and biophysical research communications. Volume 427, Number 1, October 2012, pp. 133-137, doi : 10.1016 / j.bbrc.2012.09.024 , PMID 22995305 .
- ^ W. Yi, PM Clark, DE Mason, MC Keenan, C. Hill, WA Goddard, EC Peters, EM Driggers, LC Hsieh-Wilson: Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. In: Science . Volume 337, number 6097, August 2012, pp. 975-980, doi : 10.1126 / science.1222278 , PMID 22923583 , PMC 3534962 (free full text).
- ↑ LS Gomez, P. Zancan, MC Marcondes, L. Ramos-Santos, JR Meyer-Fernandes, M. Sola-Penna, D. Da Silva: Resveratrol decreases breast cancer cell viability and glucose metabolism by inhibiting 6-phosphofructo-1- kinase. In: Biochemistry. Volume 95, Number 6, June 2013, pp. 1336-1343, doi : 10.1016 / j.biochi.2013.02.013 , PMID 23454376 .
- ↑ JL Abrantes, CM Alves, J. Costa, FC Almeida, M. Sola-Penna, CF Fontes, TM Souza: Herpes simplex type 1 activates glycolysis through engagement of the enzyme 6-phosphofructo-1-kinase (PFK-1). In: Biochimica et Biophysica Acta . Volume 1822, number 8, August 2012, pp. 1198–1206, doi : 10.1016 / j.bbadis.2012.04.011 , PMID 22542512 .