PFKFB
| Phosphofructokinase-2 / fructose-2,6-bisphosphatase | ||
|---|---|---|

|
||
| Ribbon / surface model of the dimer. The PFK domain is colored blue, the FB domain green, according to PDB 1K6M | ||
| Mass / length primary structure | 468-520 amino acids | |
| Secondary to quaternary structure | Homodimer | |
| Identifier | ||
| Gene name (s) | PFKFB1 , PFKFB2 , PFKFB3 , PFKFB4 | |
| Occurrence | ||
| Parent taxon | Eukaryotes | |
PFKFB stands for phosphofructokinase-2 / fructose-2,6-bisphosphatase (also PFK-2 / FBPase-2 ), the name for proteins that have a double enzyme function , of which there are four isoforms in humans , with the corresponding genes PFKFB1 , PFKFB2 , PFKFB3 and PFKFB4 . Depending on whether the protein has been phosphorylated , it catalyzes a reaction step of glycolysis ( EC 2.7.1.105 ) or gluconeogenesis ( EC 3.1.3.46 ). So it is a molecular switch. PFKFB1 is mainly formed in the liver and PFKFB2 in the heart, where phosphorylation of the bifunctional enzyme leads to increased kinase activity. As a result, increased glycolysis is still possible when adrenaline is released and the associated increased PKA activity. PFKFB3 does not have a preferred localization, but it is produced to a greater extent in tumors. PFKFB4 was found in the testes.
Catalyzed reactions
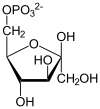
One enzyme function is the catalysis of the phosphorylation of fructose-6-phosphate to fructose-2,6-bisphosphate , an important regulator of glycolysis and gluconeogenesis. This reaction is carried out by the dephosphorylated form of the enzyme (phosphofructokinase-2). It must not be confused with the formation of fructose-1,6-bisphosphate by phosphofructokinase 1 , a central step in glycolysis.
The other function is to catalyze the splitting off of a phosphate ion from fructose-2,6-bisphosphate . This is done by the phosphorylated form of the enzyme (fructose-2,6-bisphosphatase).
The two catalyzed reactions are not reciprocal reverse reactions, since the first uses up ATP , while the second produces free phosphate. The two catalytic centers are also in different positions in the molecule.
Individual evidence
- ↑ H. Bando et al .: Phosphorylation of the 6-phosphofructo-2-kinase / fructose 2,6-bisphosphatase / PFKFB3 family of glycolytic regulators in human cancer. Clin Cancer Res . 11/16/ 2005 : 5784-5792, PMID 16115917 .
- ↑ MH Rider et al .: 6-phosphofructo-2-kinase / fructose-2,6-bisphosphatase: head-to-head with a bifunctional enzyme that controls glycolysis. Biochem J. 381 / point 3/ 2004 : 561-579. PMID 151760386 .