Telomerase
| Telomerase | ||
|---|---|---|
|
||
| Identifier | ||
| External IDs |
|
|
| Enzyme classification | ||
| EC, category | 2.7.7.49 , nucleotidyl transferase | |
| Substrate | DNA | |
| Products | Telomeres of DNA | |
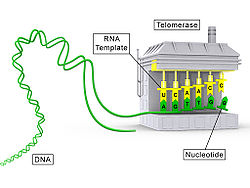
The telomerase is an enzyme of the cell nucleus , which consists of a protein - (TERT) and a long RNA is moiety (TR) and thus a ribonucleoprotein is. This enzyme restores the end pieces of the chromosomes called telomeres . The enzyme activity of telomerase can be determined using the TRAP method .
discovery
Telomerase was discovered in 1985 by the two researchers Elizabeth Blackburn and Carol Greider in the ciliate Tetrahymena . For this you were awarded the Paul Ehrlich Prize in 2009 and, together with Jack W. Szostak , the Nobel Prize in Physiology or Medicine .
features
The enzyme is a reverse transcriptase (telomerase reverse transcriptase, TERT), which uses the RNA component (telomerase RNA, TR or TERC) as a template . In humans, the RNA portion (hTERC, with h for human) is, for example, the sequence 3'- C A A U CCCAAUC-5 ', which means that the telomere is shifted by a sequence d (5'- G G T TAG- 3 ') is extended (prefix d means DNA instead of RNA).
function
The length of the telomeres is different in different species . In humans, the length is around 10 kb ( kilobases ), after several cell divisions (around 50–100) a length of around 4–6 kb can be achieved. Then the cell goes into the resting phase and no longer divides. With each cell division , a piece (approx. 100 nucleotides) of the telomeres is lost. By restoring the telomeres in certain cells, telomerase prevents the chromosomes from becoming shorter with each cell division, thus avoiding the end replication problem .
This problem arises because the DNA polymerase δ on the discontinuous DNA strand also replicates from 5 'to 3'. Starting from the RNA primer, the DNA polymerase δ synthesizes the Okazaki fragments on the discontinuous strand until it comes across an existing RNA primer. The first RNA base at the 5 'end of the primer is removed by exonuclease activity. The complete removal of the RNA primer is done by so-called flap endonucleases. The gaps in the discontinuous DNA strand are also closed with the aid of the polymerase δ and filled with deoxynucleotides . A ligase links the remaining strand breaks. Since DNA can only be replicated on the discontinuous strand as long as the binding of an RNA primer is possible, a minimal piece of the length of the RNA primer remains. Depending on the binding of the last RNA primer, this piece can be significantly longer (up to 100 nucleotides). Sequence information is thus lost at the DNA ends and the chromosome length decreases with every cell division and the associated DNA replication (see aging ).
Telomerase activity is undetectable in most normal cells . Telomerase is only active in unicellular organisms and in continuously dividing cells, such as bone marrow cells, cells of the germ line (see also germ cell ), embryonic cells (= embryonic stem cells ), stem cells and certain types of immune cells in multicellular organisms.
Telomerase is also active in cancer cells , helping them to divide infinitely often and to proliferate in the body. Non-degenerate cells can only undergo a certain number of cell divisions (so-called Hayflick limit ).
Individual evidence
- ^ CW Greider, EH Blackburn: Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. In: Cell . tape 43 , 1985, pp. 405-413 , doi : 10.1016 / 0092-8674 (85) 90170-9 , PMID 3907856 .
- ↑ G. Gavory, M. Farrow, S. Balasubramanian: Minimum length requirement of the alignment domain of human telomerase RNA to sustain catalytic activity in vitro. In: Nucleic Acids Res. Vol. 30, Issue 20, October 2002, pp. 4470-4480. PMID 12384594 , PMC 137139 (free full text), doi: 10.1093 / nar / gkf575
literature
- WK Purves, J. Markl: Biology. 7th edition. Elsevier, Spektrum Akad. Verlag, Munich 2006, ISBN 3-8274-1630-2 .
Web links
- Comprehensive telomerase database (English)